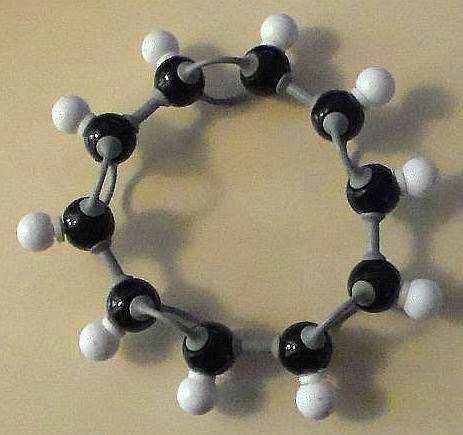
There are aromatic hydrocarbons and there are non-aromatic hydrocarbons. For basic mono-ring structures that have alternating carbon-carbon double bonds, there is a rule called Hückel’s rule that defines whether a given hydrocarbon can be aromatic or not. It might seem, at first that cyclodecapentaene (C10H10 or [10]-annulene) should be aromatic. Yet, cyclodecapentaene is not an aromatic hydrocarbon. Let’s find out why not.
A Few Simple Rules for Aromatic Behavior
- The double bonds in the ring must be conjugated (alternating). This allows for the electrons to be delocalized [in conceptualization be flipped in either a clockwise or counterclockwise direction]. This allows for the presence of what is called a ring current.
- The ring must be flat.
- The mono-ring must feature 4n + 2 π-electrons (Hückel’s rule). For example, benzene, with its n value of 1, has 4(1) + 2 = 6 π-electrons. Since it also meets requirements 1, 2, and 4, it is aromatic.
- Bond angles and atom proximities should not be so severe they nullify aromatic energy stabilization.
Why Cyclodecapentaene is not an Aromatic Hydrocarbon
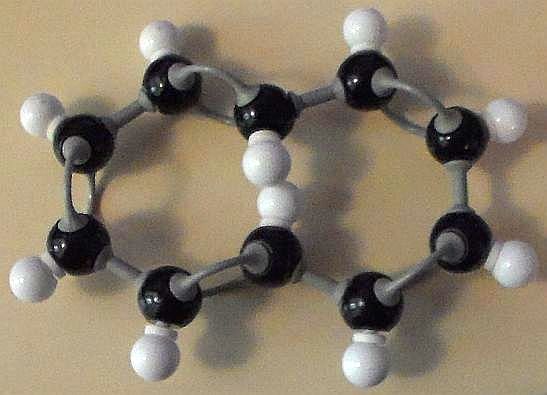
There are four basic structural shapes for cyclodecapentaene. Two of these, however, are obviously not flat. So we dismiss those two forms right off. However, the other two structures fulfill a number of the four requirements.
The rings of both the all cis- isomer (Figure 1) and the trans-cis-trans-cis-cis isomer (Figures 2 & 3) of cyclodecapentaene possess completely conjugated double bonds. Check!

Are these two forms flat? At first glance, they would seem to be. Check!
Both possess 5 double bonds or 10 π-electrons. This fulfills Hückel’s rule for n = 2. Check!
So far, based on requirements 1, 2, and 3, it looks like our hydrocarbon should be aromatic in both its isomer forms.
No. 4 Straw Breaks the Camel’s Back
Uh-oh. Both cis- and trans- isomers break rule qualification number 4.
The cis-isomer has exaggerated carbon-carbon bond angles of approximately 144°. The ideal angle for those bonds is 120°.
The trans-isomer has two hydrogen atoms that come so close together, ring flatness, though seemingly possible with a molecular model kit, is, in fact, prohibited.
Note: You might also enjoy The Aromatic Cyclopentadienyl Anion
References:
← Back to Classic Science
← Home
