In the 1900s, seeming peace was rudely interrupted by two developments in nuclear science. The first was fission. The second was fusion. Both can be used in positive ways. But rarely does mankind focus on good. What’s the difference between fission and fusion?
Fission
Fission is the dividing or splitting of something. This word well applies to the splitting of the atom. More often than not, that atom is the uranium atom. In particular, it is 235U. The 235 stands for isotope atomic weight. There are other isotopes of uranium, most notably 238U. Atoms are fissionable with a net release of energy if they have an atomic weight higher than approximately that of iron.
Fusion
Fusion behaves in the opposite manner. Fusion is defined as the joining together of two or more things to form a larger single unit. We are not talking about the formation of a chemical bond between two atoms of hydrogen that remains hydrogen, namely H₂. Rather, An example is fusing together of two atoms of hydrogen to form one single atom of helium, He. Fusion releases energy if the atomic weight of the product is lower (approximately) than that of iron.
Difference between Fission and Fusion – Examples
A few examples illustrate the difference between fission and fusion. They are listed below for comparison purposes.
235U + n → 236U → 144Ba + 89Kr +3n +177 MeV
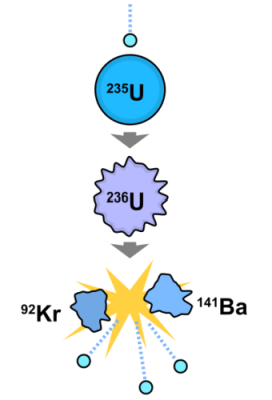
The above fission reaction says one atom of Uranium 235 smacked by a neutron produces one atom of Uranium 236. This is very unstable. It quickly splits into an atom of barium (atomic weight 144) and an atom of krypton (atomic weight 89), plus three neutrons and 177 mega-electron volts of energy. The three neutrons promote further reaction if additional uranium atoms are present.
Another fission reaction is
⁶⁰Co₂₇ (unstable) → ⁶⁰Ni₂₈ + e⁻ + energy (including γ-rays)
What’s this? The atomic weights of the cobalt and the nickel are the same (atomic weight 60 → 60). The atomic number of the nickel is higher (28, rather than 27 or less) than that of the cobalt! How is this fission? The cobalt-60 with atomic number 27 was produced artificially. It is unstable. One of the nucleus neutrons splits apart, giving a proton that stays within the nucleus (raising the proton number from 27 to 28). The electron exits the nucleus. Since the cobalt atom produces the nickel atom PLUS an electron (two particles), the reaction is fission.
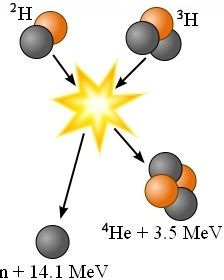
The following fusion reaction reads one atom of deuterium fused with one atom of tritium produces one atom of helium having two neutrons and two protons in its nucleus plus a free neutron plus 17.6 mega-electron volts of energy.
²H + ³H → ⁴He + n + 17.6 MeV
Fusion and Fundamental Forces
In fission, it is frequently bombardment of a large molecule by a smaller particle that initiates reaction. If a number of high-energy smaller particles are produced that can perpetuate the reaction, so much the better. However, what is required to initiate fusion? Fusion involves an increase in size of the nucleus.
Neutrons and or protons must be added. But positive repels positive. Or so electrostatic force, a fundamental force, dictates. But there are four commonly accepted fundamental forces of nature. One of those is nuclear strong force. If nuclear particles can be brought into close enough proximity for the needed time, strong force prevails over electrostatic force and fusion results.
1 deuterium is hydrogen with 1 electron orbiting a nucleus containing 1 proton and 1 neutron.
2 tritium is a radioactive hydrogen isotope with 1 electron orbiting a nucleus containing 1 proton and 2 neutrons.
Note: You might also enjoy Total Energy in One Hydrogen Atom
References:
- Georgia State University Hyper-Physics: Uranium 235 Fission
- Institute for Fusion Studies: Theory Group
← Back to Classic Science
← Home
