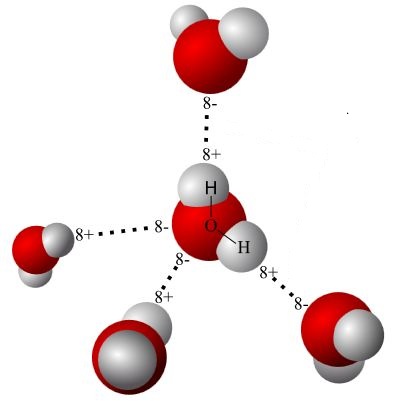
Mickey the Dipole? Everyone knows H₂O is the chemical formula for water. H stands for hydrogen. O stands for oxygen. The water molecule is made from two atoms of hydrogen and one atom of oxygen.
Hydrogen atoms have one proton and one electron. Ordinary oxygen atoms have eight protons and eight neutrons and eight electrons. For the purposes of this discussion, we can forget the protons and the neutrons.
Oxygen has a thirst for electrons. Hydrogen is “happy” to give up its electron. The reaction of hydrogen with oxygen (each of which exist as a pair) is,
2 H2 + O2 → 2 H2O
The hydrogen parts are positive (H⁺). The oxygen part is negative (O–).
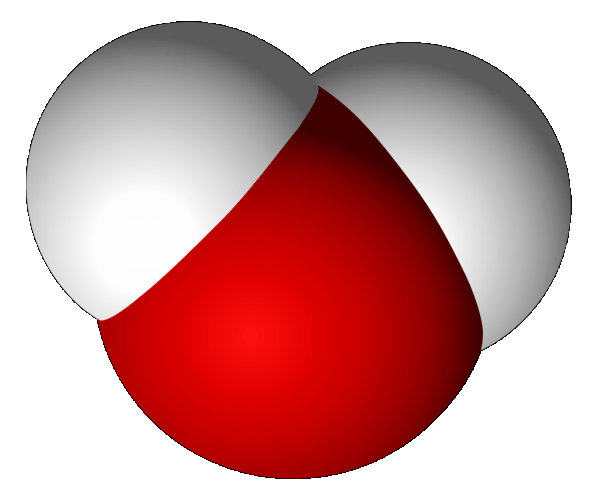
One might think water would assume a linear geometry H—O—H. But it does not. It is bent. Does it matter? Yes, it is of great importance. It is one reason life exists on our planet!
Questions and Mickey the Dipole
The oxygen atom in a water molecule is relatively large. The hydrogen atoms are comparatively small. Water is shaped like the head of a mouse. Thus, it is nicknamed Mickey the Dipole. Questions arise:
What is a dipole?
Why are both hydrogen ears on the same side of the “head”?
Questions Answered
Magnets have two poles: north and south. In atoms there are positive charges and negative charges. Some molecules have positive and negative portions.
The two “ears” of Mickey the Dipole are positive hydrogen atoms, The head is a negative oxygen atom. It would be logical to suspect the two ears would naturally be 180° opposed to each other, lying on exactly opposite sides of the oxygen atom. Since they are not, what is the explanation?
It is because there are electrons called lone pairs that are not involved in the bonding of hydrogen to oxygen. They lie in a position about the oxygen that are tetrahedral to the hydrogen atoms.
Hydrogen Bonding
Since water is dipolar, it forms weak bonds (hydrogen bonds) with other molecules. These molecules can be more water molecules or other molecules. Hydrogen bonds have less than a full charge in magnitude. Nevertheless, they are very influential.
Many life processes succeed because of hydrogen bonding. For instance, thanks to hydrogen bonding, as ice forms, it floats rather than sinks. The floating layer acts as an insulating blanket over the water beneath. Living things beneath that layer survive.
Hydrogen bonds impart structural folding to protein complexes. This folding is essential to proper function. For an improved understanding of this, visit the University of California Irvine’s page entitled General Principles of Membrane Protein Folding and Stability.
Note:You might also enjoy the article Polymeric Water Clusters
References:

I have read claims that the “dipolarity” of water can be reversed, this is a claim that sellers of electrical osmosis damp proof systems make. Is this possible and if so how will this reverse capillary action?
There’s no such thing as “reversing” a dipole.