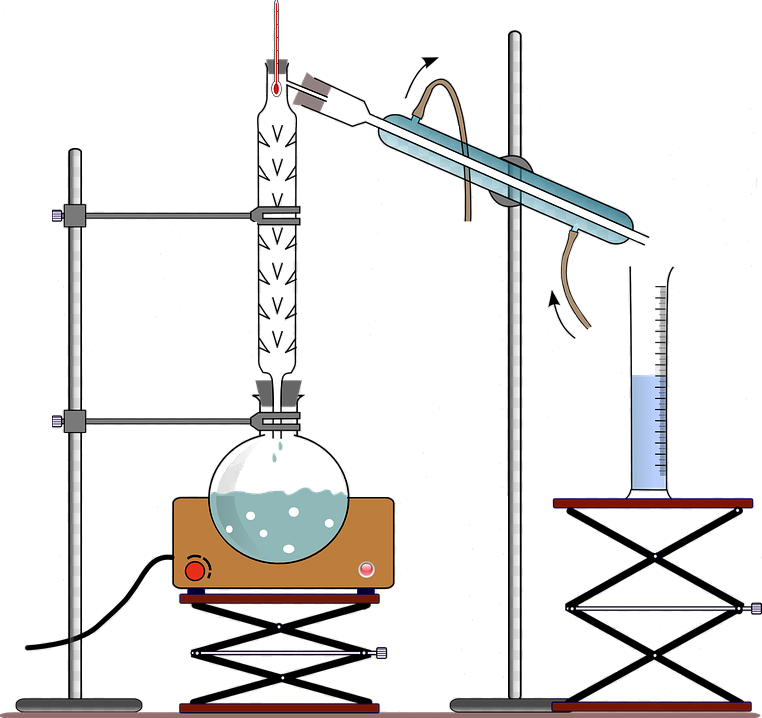
Preparing an alkyl halide was one of the earliest of my home lab experiences. First, allow me to give you a little background.
In the 8th grade, we were instructed by our English teacher, Mrs. Best, to write a career report about our intended future employment. My guess is most of us had not thought about that. At least I hadn’t. So I chose astronomy. I mentioned this to Mom. “No,” she said, “you will not become an astronomer. Astronomers don’t make any money.” I tried to talk her out of it, but eventually I gave up.
“Chemistry,” I said. Mom agreed that was acceptable. One of her friends had a chemistry background. So in the ’70s I obtained my BS in chemistry. While still in high school, I constructed a home chemistry lab in the basement of our house. About five or six years later, sometime between about 1965 and 1968, I decided to synthesize an alkyl halide, in particular an alkyl bromide.
An Alkyl Halide
What is an alkyl halide? As its name suggests, it is a compound derived from an alkane and a halogen atom, in this case a bromine atom. An alkane consists of carbon (C) and hydrogen (H) atoms. Hydrogen, valence 1; carbon valence 4. The simplest alkane is methane CH₄.
The second simplest alkane can be made from two methane molecules, stripping one hydrogen from each, to produce ethane, CH₃-CH₃. The third simplest alkane is propane, CH₃-CH₂-CH₃. But these are alkanes. What is alkyl? Simply remove at least one hydrogen atom and it becomes an alkyl group. Whether the alkyl group is methyl, ethyl, propyl, or something else, it is often written (generically) as R-.
Now alkanes are slow to react chemically. So sometimes an alkyl alcohol is used to make an alkyl halide. The reaction for the formation of an alkyl bromide from the alcohol is,
R-OH + HBr → R-Br + H₂O
Thus n-butyl alcohol plus hydrobromic acid in the presence of sulfuric acid produces n-butyl bromide.
CH₃CH₂CH₂CH₂-OH + HBr → CH₃CH₂CH₂CH₂-Br + H₂O
The above generic reaction and example involve a primary alcohol. Notice the straight chain of carbon atoms (with attached hydrogen atoms). The -OH group is attached to the end of the straight chain in the above reaction. It is a primary alcohol.
Reaction Mechanism
The basic mechanism involving sulfuric acid and the above primary alcohol may be written
CH₃CH₂CH₂CH₂-OH + H⁺ → CH₃CH₂CH₂CH₂⁺ + H₂O
CH₃CH₂CH₂CH₂⁺ + HBr → CH₃CH₂CH₂CH₂Br + H⁺
The generation of another hydrogen ion should guarantee the continuance of the reaction process. Notice that the above primary alcohol is saturated. That is, there are no double bonds, there is no unsaturation.
Allyl Bromide
Refluxing allyl alcohol (note: not the generic alkyl, but a specific alcohol, allyl) with hydrobromic acid in the presence of a little sulfuric acid primarily produces allyl bromide.
R- is, in this instance, the straight-chain H₂C=CH-CH₂-.
H₂C=CH-CH₂-OH + H⁺ → H₂C=CH-CH₂⁺ + H₂O
H₂C=CH-CH₂⁺ + HBr → H₂C=CH-CHBr + H⁺
The double bond complicates matter slightly. It might be suspected mere substitution does not occur to produce pure allyl bromide. The double bond suggests at least some addition reactions occur, producing H₃CHBrCH₂OH and H₃CHBrCH₂Br and traces of other compounds.
Being young and assuming an air of intelligence, I started the reaction and went upstairs to obtain a snack. When I came back downstairs, I was shocked, even horrified! What had gone wrong? I quickly turned the reaction off, opened the window, and left the room.
The Unanticipated
I remembered alkyl bromides having an aroma that is not unpleasant. I expected this would be true of allyl bromide. But consider what Wikipedia says of the stuff… “Physically, allyl bromide is a clear liquid with an intense, acrid, and persistent smell.”
Then questions flooded my mind. What if my parents discovered I’d done this? What of the pets? Fortunately, my parents were not at home. I opened all the windows and let the pets out. I left for a friend’s house and returned later. Joy of joys, my parents had not come home and the smell was gone. I closed the windows, let the pets in, and never breathed a word to my parents. But I’ve never forgotten the time I decided to prepare an alkyl halide.
Note: You might also enjoy I Follow Dmitri Mendeleev’s Recipe for Chromyl Chloride Preparation
References:
← Back to Classic Science
← Home

For those who enjoy terminology, when I was young, the carbon chain ending with a positive charge in place of a hydrogen atom was called a carbonium ion. Generally speaking, today it is called a carbocation. One must keep up with change.
You were lucky! My father was a pharmacist and used to let me try various experiments under his supervision. One was on friction and how to strike a non-safety match on glass (but don’t tell your mother!). Lucky I didn’t end up an arsonist. Nowadays, many matches are of the safety type and you can’t do that with them. Youth is a time for experimentation but some is safer than others. A man I know used to do the same as you. One time he tried floating an alkane lamp (I think it was – an old bicycle lamp) down a sewer and ended up exploding the sewer! He never grew up (he still hasn’t) but his kids and mine loved playing together in all sorts of odd places. One time he told one of my sons he could polish his nails with a rotating disk – nearly took his finger off!
Meg, This comment made my day. As to the match, I expect rubbing upon glass generated more heat than the match head would have otherwise received, igniting it. I’m glad I wasn’t a chemist wannabe during the time of the discovery of fluorine. Many died in the process.