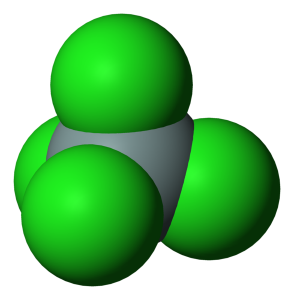
The empirical formula of the compound silicon tetrachloride is SiCl₄.
The molecule possesses tetrahedral symmetry. This means the atom of silicon is located at the center and the four atoms of chlorine are located at the four corners of a tetrahedron.
Each chlorine atom is strongly electronegative, so the molecule of SiCl₄ strongly draws electrons. The core, silicon atom is insignificantly electronegative.
Elemental Electronegativity
Electronegativity is the tendency of an atom or other particle to attract electrons. It is high when an atom is small and outer electrons are least shielded from the positive nucleus. This makes fluorine the most electronegative of elements. Conversely, the alkali metal astatine, is least electronegative. Chlorine, while not so much so as fluorine, is still very electronegative.
Molecular Electronegativity
A molecule of silicon tetrachloride is larger than any atom. Yet, the four chlorine atoms it contains are symmetrical about the silicon atom and act like they were electrons. The silicon atom acts like a large nucleus. A molecule of SiCl₄, then, is much like a very large electronegative element.
Silicon Tetrachloride – Conclusions
What can be conclude from this? That sometimes a small object can mimic a much larger object. In this instance, the properties of a molecule are similar to those of an atom.
Note: You might also enjoy Inert Gas Compounds?
References:
