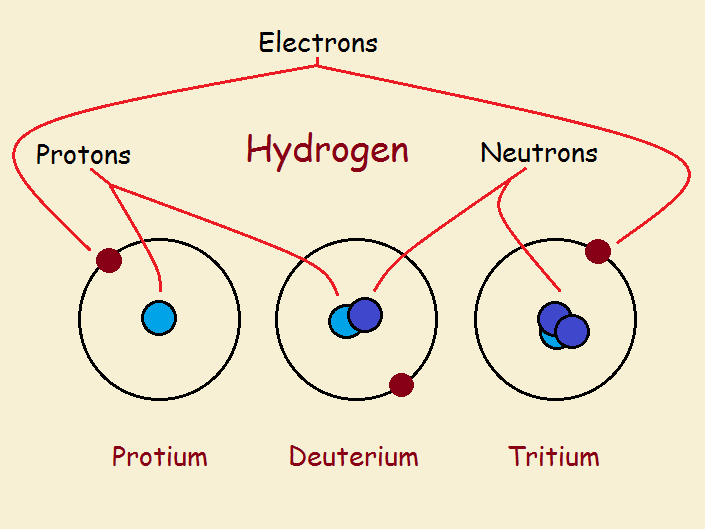
 Elements are the building blocks of a chemist’s world. The first and simplest element is hydrogen, H. It is a gas at room temperature. A molecule of hydrogen gas consists of two joined atoms. However, we will discuss the lone atoms, which exist in three varieties: protium, deuterium, and tritium.
Elements are the building blocks of a chemist’s world. The first and simplest element is hydrogen, H. It is a gas at room temperature. A molecule of hydrogen gas consists of two joined atoms. However, we will discuss the lone atoms, which exist in three varieties: protium, deuterium, and tritium.
All hydrogen atoms have an atomic number of 1. This means the central core or nucleus of any of the three varieties of hydrogen contains just 1 proton. All hydrogen atoms have 1 electron in an orbital outside the nucleus.
What makes the three varieties different? The nucleus of hydrogen can include in addition to the proton, zero, one, or two neutrons.
Protium
Hydrogen without any neutron is protium. Hydrogen with one neutron is deuterium. Hydrogen with two neutrons is tritium. Tritium alone of the three is less than completely stable. It is radioactive. The ratios of these three forms of hydrogen in the vicinity of the earth is approximately:
99.98% H-1 (protium)
0.016% H-2 (deuterium)
<0.01% H-3 (tritium)

As can be seen from the numbers, the most important isotope is protium or H-1. However, there are special applications for deuterium.
Deuterium
Deuterium was discovered in 1931. Since a neutron weighs just a bit more than a proton, deuterium is slightly more than twice as heavy as protium. Two atoms of deuterium (sometimes called heavy hydrogen) combined with one of oxygen is called heavy water. Oxygen is the same in both regular water and heavy water. It possesses the bulk of the mass. So heavy water is only about 10% heavier than drinking water. Chemically heavy water resembles regular water. But there are important differences.

Percent wise, the difference in mass between H-1 and H-2 is much greater than the difference between isotopes of other elements. Mass affects bond length. Bond length affects other properties. Because water is so important to life, heavy hydrogen is very useful in biological research.
Nuclear magnetic resonance (NMR) and infrared (IR) spectroscopic properties differ considerably between protium and deuterium. So labeling organic compounds with one or more deuterium atoms can help in the study of organic reactions. In NMR, various deuterated solvents prove most useful in structure elucidation.
Tritium

Tritium is considered a waste product in nuclear reactions. It presents health risks if it reaches an underground water supply. For instance, in the US there was an important tritium leak. See this document from the Brookhaven National Laboratory (BNL). Sometimes, however, tritium is deliberately employed in the armaments business in fission bombs and in the fission component of fusion bombs.
Afterword: If you are particularly interested in these isotopes of hydrogen, and perhaps isotopes in general, you may find this article particularly interesting: Isotopic Varieties of H2O: How Many Kinds of Water are In Your Glass?
Note: You might also enjoy Total Energy in One Hydrogen Atom
References:
← Back to Classic Science
← Home

I am wondering if heavy water would be safe to drink or not.
I wonder at what temperature and pressure does a mole of each isotope of gaseous hydrogen undergo nuclear fusion, and how much energy would one mole of each isotope produce under those conditions?
Also, if you used deuterium or tritium in a fuel cell, would you get more or less energy than if you used protium?
Not sure if you still need this answered, but heavy water is fine to drink in small or average amounts! It only starts becoming toxic when it’s consumed at about 10% of your body weight.
Nick, I’m pretty sure water is fine to drink, water that’s heavy has everything to do with the amount present. So, essentially, your question is the same as asking if dumping a pond’s-worth of water down your gullet at once is safe. It is not.