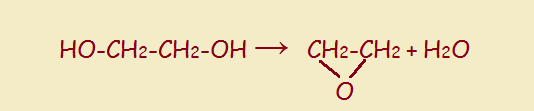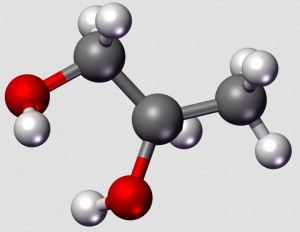
In our food, we expect to find ingredients of the earth, sea, and air—ingredients that occur naturally without any finagling from humans. Grains, vegetables, fruits, meats, fish, fowl, and cheese are all welcome. But go to your neighborhood grocery and pick up any package of prepared food product, and you are likely to be assaulted with a huge array of substances that sound nothing like these ingredients. That array will include thickeners, anti-caking agents, preservatives, fungus preventatives, and so forth. Yet another ingredient may be some form or other of glycol. What is that?
Simply put, a glycol is a double alcohol—two adjacent carbon atoms each has a pendant hydroxyl group. The structure is seen to the right, below:
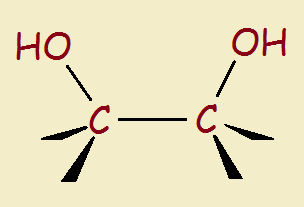
Although the glycol linkage occurs in nature, glycols aren’t ingredients ordinarily found in a homeowner’s kitchen cupboard. Why do manufacturers sometimes add a glycol to foods or beverages? There are a number of reasons.
Why Put Glycol in Your Food?
Dow Chemical explains its PuraGuard™ PG USP/EP propylene glycol product may be used as a humectant, a stabilizer, as a solvent in flavor solutions and extractions, as a plasticizer and softener, and as a heat transfer liquid for freezing and chilling applications.
How Do You Respond?
How do you respond to the presence of such chemicals in your foodstuffs? Some might say, I don’t like it at all. I don’t want anything unnatural to enter my body. Others may swing the other way and—pointing a finger at ‘purists’—they may identify them with ‘animal lovers’ and ‘tree huggers.’ “Get used to it,” they may say—it’s part of the modern world.
Government agencies, such as the Food and Drug Administration specify what and how much of any substance may go into the food supply of Americans. Although they feel what they allow is acceptable, is that what you think and how you feel? If your shopping cart holds foodstuffs containing additives such as glycol, you have made your statement. What you do speaks louder than what you say.
Chemistry Extra Credit
Just as an alcohol can lose water (be dehydrated) to form an ether—for instance, diethyl ether,
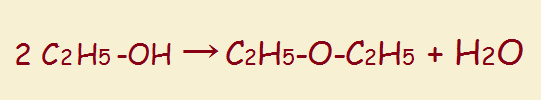
So, too, a glycol can lose water. In this instance, it often takes only one molecule, which can be dehydrated internally. Thus,
The oxygen-containing ring produced by this dehydration is called an epoxide. An epoxide is a cyclic ether.
Note: You might also enjoy Organic Chemistry: What is a Lactam?
References:
- FDA: CFR Title 21 Sec. 184.1666 Propylene glycol
- WebMD: Drugs & Medications – Polyethylene Glycol 3350 Oral