A substance exhibiting pyrophoricity (including certain metals) reacts quickly with oxygen, producing heat, and bursting into flames. Alkali metals are pyrophoric, but there are other pyrophoric metals as well, given the proper conditions. Otherwise, they are stable and may be put to a number of uses without fear of bursting into flame.
The overall principle is a simple one: metals are electropositive. Oxygen is electronegative. Almost all metals will react with oxygen to some extent. As a metal oxidizes, the reaction releases a little heat energy. If the heat cannot dissipate, it builds up. This speeds further oxidation, increasing the heat yet more. The metal may burst into flames.
Those “Other” Metals
Some alkaline earth metals such as calcium, and a few other transition metals such as iron, thorium, and uranium, exhibit pyrophoric behavior. Now these metals don’t ordinarily burst out in flame. Why not? It’s because it is a matter of degree.
A machinist uses a grinder on a piece of metal. Do you see the sparks? Those sparks are metallic in nature. The hard grinding stone does two things: it tears off tiny bits of metal, breaking metal to metal bonds. It also produces friction. Both of these produce heat. Heat increases the rate of reaction of metal with oxygen in the air.
Another factor is the size of the particles. Although they are not spherical in shape, it is easy to show mathematically why reactivity increases so greatly.
By Way of Illustration
As a sphere approaches the size of an atom (say of iron) the increase in oxygen to metal ratio is so much improved, reaction under heat is assured. To illustrate how that works: have you ever tried to burn a log in a fire place with a match? It doesn’t happen unless you first use kindling wood, right? And if very tiny twigs are included, it takes no time at all to make a blazing fire.
Uranium
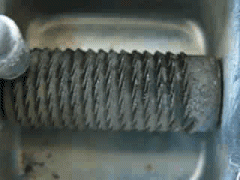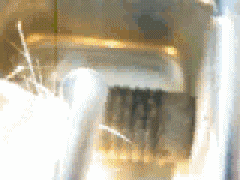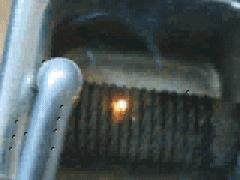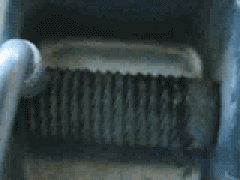
Uranium metal exhibits pyrophoricity. Notice what happens when a uranium rod is passed across a metal file in this YouTube video:
Pyrophoric Alloys
Some metals are reactive enough they are pyrophoric in bulk. One of these is the rare earth metal, cerium. Combining cerium with iron produces the alloy ferrocerium. This is used in very cost effective strikers, used to light a welding torch or a Bunsen burner (see the animated GIF-image associated with this article).
Note: You might also enjoy Why Sparks from Steel and Flint?
References:
← Back to Classic Science
← Home

That was very interesting. I hadn’t thought about strikers before.
Small amounts of pyrophoric liquids are often supplied in a glass bottle with a PTFE (Teflon)-lined septum. Larger amounts are supplied in metal tanks similar to gas cylinders, designed so a needle can fit through the valve opening. A syringe, carefully dried and flushed of air with an inert gas, is used to extract the liquid from its container.