Na + Cl → NaCl
We usually write the sodium molecule exactly as it appears above. But occasionally, we write it out in greater detail, as Na–Cl. The dash pictures a single bond between constituent atoms. The bond is a single bond because sodium’s valency (or desire to react if you will) is positive one, or +1. Chlorine’s is negative one, or –1.
Double Bonds
Some atoms exhibit a higher valency. Carbon atoms in organic compounds are most often assigned a valency of 4. Carbon exhibits another marvelous feature. It can bond to itself extensively. In such instances, the 4 valency is not considered positive or negative. Both single and multiple bonds can form. As an example, we choose ethane, ethene (AKA ethylene), and ethyne (AKA acetylene).
H₃C–CH₃ [ethane]
H₂C=CH₂ [ethene, AKA ethylene]
HC≡CH [ethyne, AKA acetylene]
Notice the single bond, double bond, and triple bond, successively.
Cis and Trans Double Bonds
In their most common uses, which are also the simplest uses, the prefix cis means same and the prefix trans means across. Especially is this in reference to a double bond. Take note of the example provided in the image, above.
After looking at it, someone might say, “But aren’t there four different 2-butenes, rather than just the two pictured, the cis and the trans?
No. There are not. Flip the two drawn either top to bottom or left-to-right and they may appear different. But on closer examination, it is apparent they are the same, cis-2- and trans-2- butene.
Can a Molecule be Both?
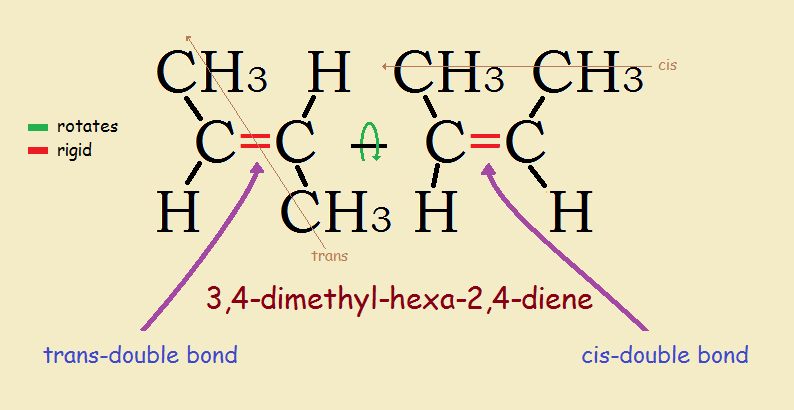
If a molecule has more than one double bond, the molecule can have both cis and trans bonds. For example, 3,4-dimethyl-hexa-2,4-diene, with two, has one cis-double bond and one trans-double bond (see topmost image).
The difference between cis and trans is not merely of intellectual value. Life chemistry requires very specific chemical structures. And some of those structures have many double bonds in them, the geometry of each being important.
Stick Rendering Simplifies Interpretation
Organic chemistry contributes about as many words and phrases to the language as the law does! One method of drawing molecules greatly aids in the recognition of cis and trans double bonds. It is stick rendering. In stick rendering, hydrogen atoms are pretty much ignored. Carbon atoms singly bonded to carbon atoms are given a straight-line segment. Thus ethane is a straight line segment only. Propane is a V. The final image associated with this article pictures cis and trans 2 pentene molecules. See how simple it all is?
Note: You might also enjoy Keto Enol Tautomerism
← Back to Classic Science
← Home