 Is the term keto enol tautomerism familiar to you? If not, give attention to the ensuing discussion.
Is the term keto enol tautomerism familiar to you? If not, give attention to the ensuing discussion.
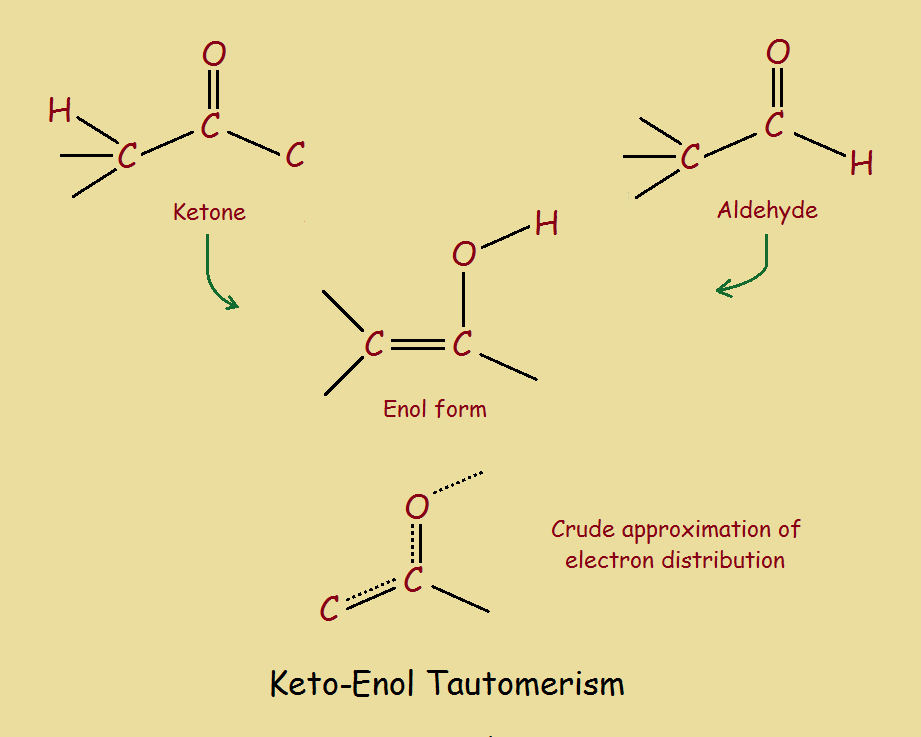
Organic chemistry would be of no value if not for multiple bonds and functional groups. Such groups impart the reactivity needed to sustain life. One attachment is carbonyl (–C=O).
Tautomerism
Given the right environment, atoms of a carbonyl group tautomerize. That is, they can exchange a proton (hydrogen). In so doing a π-bond is formed or disappears. The carbonyl or keto form assumes the alkene-alcohol or enol form, or vice versa. For acetone, the two forms are,
CH3–(C=O)–CH3 keto form
CH2=C(OH)–CH3 enol form
See the image above.
Both Aldehydes and Ketones Participate
A ketone has its carbonyl carbon atom linked to two other carbon atoms. An aldehyde has its carbonyl carbon atom linked to one carbon atom and one hydrogen atom. For examples, again see the image.
Although an aldehyde is not a ketone, the reaction is similar. It too is said to undergo keto enol tautomerism. In any instance, a proton is transferred to or from oxygen.
Keto Enol Tautomerism Is Important
Since the exchange is back and forth, it might seem keto enol tautomerism is of no importance. But it is! When the keto form prevails, the C=O double bond is subject to addition. If can convert to a single bond. Tautomerism determines the mode of attack and the end result.
Which Form is More Stable
In general, the keto form is more stable. Oxygen’s electronegativity is satisfied. The enol form is favored by the presence of hydronium ion. This can be added in the form of a trace of mineral acid. Often hydrochloric or sulfuric acid is used.
Note: You might also enjoy Imidazole Synthesis and Chemistry
References:
← Back to Classic Science
← Home
