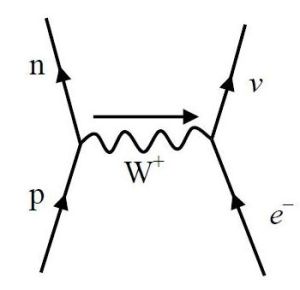
There is not one, but two processes, called electron or e– capture.
Atoms can be visualized as two parts. The central nucleus, an inner core of protons plus neutrons, and an external shell of electrons traveling in their various orbitals.
Ordinary chemical processes involve the making or breaking of bonds between two or more atoms. These bonds involve the electrons and the openings that hold them.
The physics process of e– capture is the penetration by an inner shell electron into the nucleus of a neutron rich atom. Absorption by one of the protons replaces it with a neutron. At the same time, a neutrino and an X-ray photon are emitted.
What Defines an Element?
Element identity is determined by the proton count in the nucleus. Hydrogen has one proton. Helium has two. Chlorine has seventeen. There are a few more than 100 elements in all. While the number of neutrons has no bearing on an element’s identity, it does affect an its properties to a degree. Most notably it affects nuclear stability.
Since the number of neutrons in any element’s nucleus can vary, it is not wrong to suggest there are a number of varieties of the same element. These are called isotopes. Now a neutron capture does not produce a mere change in isotope. It produces a change in element identity. The number of protons in the nucleus has been lowered.
Decay
Since capture usually involves the first electron shell (AKA the K-shell), the process is also called K-capture. K-capture is not the only way unstable isotopes decay. There is also beta decay. This process requires greater initiation energy.
Elements with a weight less than the more stable isotopes usually decay by the capture process. Those heavier than the more stable isotopes tend to undergo beta decay.
A Different Capture Process
There is another unrelated process that might be confused with the process described above. Organic compounds such as large biomolecules can be bombarded with electrons in a form of mass spectrometry. These molecules undergo a kind of capture to produce odd electron fragments.
Biomolecular e– capture dissociation was first described by R. A. Zubarev of the Cornell University’s McLafferty group in the 1998 article, Electron Capture Dissociation of Multiply Charged Protein Cations – a Nonergodic Process.
Note: You might also enjoy Factors that Complicate Atomic Mass Determination
References:
- IUPAC Gold Book: Electron Capture
- Georgia State University: Hyperphysics: Electron Capture
- Purdue University: Electron Capture
- Boston University School of Medicine: Electron Capture Dissociation
