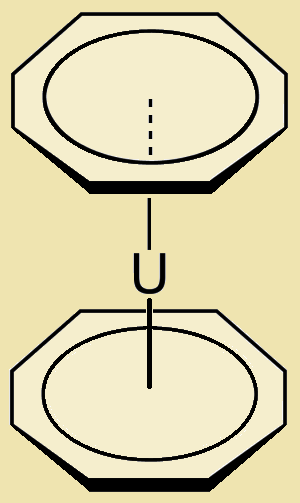
 Uranocene, U(C₈H₈)₂, or biscyclooctatetraenyl uranium, is a most interesting aromatic organometallic sandwich compound.1 Notice its dumbbell-like shape. By no means is its shape uranocene’s only claim to fame.
Uranocene, U(C₈H₈)₂, or biscyclooctatetraenyl uranium, is a most interesting aromatic organometallic sandwich compound.1 Notice its dumbbell-like shape. By no means is its shape uranocene’s only claim to fame.
But before we get into that, it would be helpful to explain terms. We can guess why it’s an organometallic compound, just as we can guess why it’s called a sandwich compound. Let us, however, discuss what we mean by the term aromatic.
Aromaticity in a Nutshell
An aromatic structure is a molecule or ion that is cyclic and flat and that incorporates 4n+2 conjugated (alternating) sp2 (double) bonds, where n is some small integer. It displays increased stability over non-aromatic structures. Despite possessing double bonds, aromatic structures tend to undergo substitution rather than addition reactions.
It requires considerable energy to disrupt aromatic bonding.
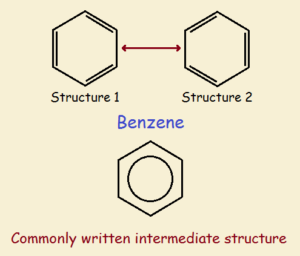
Multiple resonant structures can be drawn that describe the structure equally well. Thus, for benzene, two primary structures may be drawn as shown in the figure below. Flip the structure on the left and you obtain the one on the right. The third sketch portrays benzene as it actually is—a kind of intermediate. The spread out charge stabilizes the structure.
Annulenes
Although not all aromatic compounds contain just one ring, many do. Even-numbered annulenes are defined as conjugated monocyclic hydrocarbon rings with the chemical formula CnHn, the n being an even number. Thus we can write Benzene as [6]annulene. Cyclooctatetraene or C8H8 is [8]annulene.
Odd-numbered annulenes are similarly defined as conjugated monocyclic hydrocarbon rings, but possessing the chemical formula CnHn+1. One important example is cyclopentadiene or [5]annulene, C5H6. Another is cycloheptatriene or [7]annulene, C7H8. We will center our discussion on [5]annulene and [8]annulene.
It is important to remember that most annulenes are not aromatic. In fact, [8]annulene, if it assumed planarity, would be antiaromatic. We will see later how in uranocene, all that is changed!
The Historic Molecule Ferrocene
As has so often been the case in the past2, the discovery of ferrocene was largely accidental. In an attempt to prepare the organic compound fulvalene, an unexpected orange powder was obtained that proved to be ferrocene. See the illustration for the reaction details, both the anticipated reaction and the actual reaction obtained.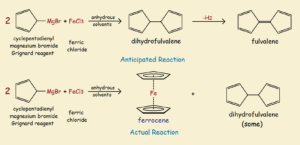
Why Uranium?
So what made scientists pursue uranium for the derivation of an organometallic compound? Was it due to purely theoretical interest, or did it go beyond that? In fact, as is sadly so often the case, it was influenced by military considerations.
We quote briefly from the Organometallics journal, cited in the References section…
“During World War II the need for uranium compounds that were volatile and thermally stable for use in 235U/238U isotope separation in the Manhattan Project made organic derivatives of uranium of interest.”
As is often the case when pursuing complicated chemical syntheses, it took time to develop uranocene. In fact, a successful synthesis was first described in 1968. The reaction representing the chemistry is written:
K + C8H8 → K2[C8H8]
K2[C8H8] + UCl4 → U[C8H8]2 + KCl
That uranocene is aromatic is attested to by the planarity, the flatness, of the cyclooctatetraenide rings.
Uranocene in Retrospect
Uranocene is quite unstable in air and is said to serve no practical purpose. Does this mean, then, that the production of this uranium metallocene was ill-advised, a waste of time? Not really. To quote the journal previously cited, once again, we read…
“The discovery of uranocene in 1968 was a milestone in organouranium chemistry. Uranocene attracted much attention in the general organometallic community…”
In fact, the successful synthesis of uranocene paved the way in the pursuit of further organoactinide chemistry3.
1 Sometimes written, U(cot)2, “cot” standing for cyclooctatetraene.
2 As, for example, in the cases of penicillin and Teflon.
3 The actinides consisting of the having atomic numbers ranging from 89 (actinium) to 103 (Lawrencium).
Note: You might also enjoy The Fascinating Acetylacetone Molecule and Its Acetylacetonate Salts
References:
- Michigan State University: Aromaticity and Hückel’s Rule
- StackExchange Chemistry: In Hückel’s rule, can n be any integer?
- ACS: Organometallics 2004, 23, 3562-3583: Uranocene. The First Member of a New Class of Organometallic Derivatives of the f Elements [PDF]

Yes, military money can send research in directions it might not normally have gone. 🙁