
“Be careful if you will be driving this evening,” the weather forecaster declares. “The road will be a sheet of ice.” Whether tar and gravel, asphalt, or concrete, roads most of the year are not slippery. Yet come winter, those roads can be dangerously slippery. What makes ice and icy roads so slippery?
What Makes Ice Slippery
One line of thinking is that ice is slippery because water (H₂O) expands as it freezes. When a heavy object rests upon a sheet of ice, the pressure imparts energy to the molecules immediately beneath the weight, pressing them down, melting it. The water acts much like a lubricant, making the ice slippery.
In truth, if the ice is reasonably close to its melting point, a microscopic layer might melt this way. But computational data suggests this explanation is unsatisfactory. A seemingly better idea is that moving a heavy weight across ice generates friction. That friction produces heat that, again, melts a microscopic layer, making the ice slippery. Again, not a satisfying answer.
A recent and more intellectually pleasing concept is that the outer ice layer molecules, unlike the inner molecules, are not completely surrounded by “fellow” molecules. As a result, this outer layer seeks to rearrange its surface bonds to achieve stability. This behavior is typical of a liquid.
But could there be another factor just a little different from this one that plays a role? To understand this possible factor, let’s consider a substance similar to water yet different. That substance is a gas—hydrogen sulfide (H₂S).
Hydrogen Sulfide –vs.– Water
Oxygen is an essential part of ordinary combustion, whether it is within the body to turn food to heat energy or outside the body to burn wood or another fuel such as propane. At room temperature, water is a liquid.
Sulfur can also be used in combustion. Who hasn’t heard the words “fire and brimstone” used in connection with destruction? Brimstone is another name for sulfur. Sulfur was used to burn garbage. Sprinkled between layers of garbage, it kept the fires going.
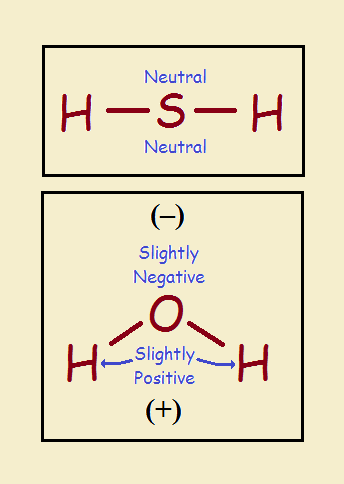
Now oxygen is a gas and sulfur is a solid. Why is it water is a liquid and hydrogen sulfide is a gas? Wouldn’t it seem more logical the other way around? The reason it isn’t is because hydrogen sulfide is linear or straight. Water is bent. See the illustration.
Hydrogen sulfide is the same right-to-left. That is, it is symmetrical. It exhibits uniform charge distribution. It is non-polar. Water is polar. Polar molecules tend to line up according to their intermolecular charge geometries. Non-polar molecules act more independently. The independence causes hydrogen sulfide to remain a gas.
Back to Our Discussion
So what may be another factor that makes ice slippery and led to our discussion of bent water versus straight hydrogen sulfide? Well, what if, in placing weight on a sheet of ice, at least some of the surface molecules are forced to straighten? Might they not act like hydrogen sulfide, some even vaporizing, escaping into the atmosphere? The gas vapor might impart a slip to the firm grip on the ice. This sounds like a re-write of the first suggestion, doesn’t it?
Such an eventuality could occur only on the surface, adding to any slipperiness imparted by one or more of the other factors previously discussed, and any additional factors not yet discovered. If anyone can present additional insight on this, please feel free to discuss it in the comments section or via the contact us section at the bottom of the page.
References:
- Ohio State University: Why is Ice Slippery? By Robert Rosenberg
- University of Wisconsin Green Bay: Ice Structure

Sounds like ice is a bit more complicated than I realized.
Interesting idea.