Matter and the atoms that make it up consists of electrons, protons, and neutrons. The center or nucleus of each atom consists of at least one positively charged proton.
Usually the nucleus includes neutrons, which are uncharged. Negative electrons are found in orbitals well outside the nucleus. Their number equals the number of protons in the nucleus. Such atoms have a net charge of zero.
Elements
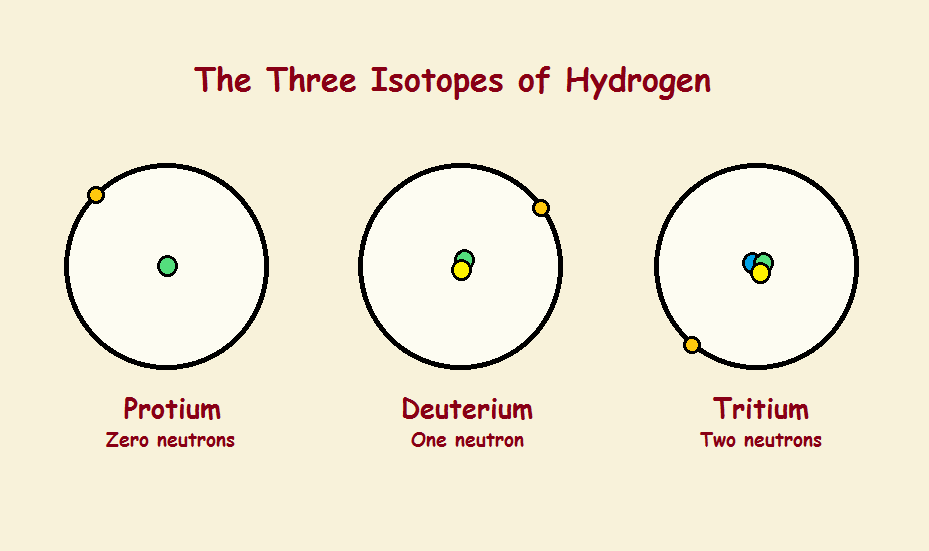
The number of protons determines what element an atom is. One proton identifies hydrogen, two protons helium, three lithium, four beryllium, five boron, six carbon, and so on. Each of these elements possesses a unique chemistry.
The quantity of protons equals the quantity of electrons, so everything’s set, right? Well, no! The number of neutrons in all the elements can vary. For instance, stable helium atoms can (and do) have 1, 2, or even 3 neutrons in its nucleus. Carbon atoms contain 6, 7, or 8 neutrons. Each of these variants is called an isotope. Each isotope behaves slightly differently. There are isotope effects.
Atomic Mass
Some speak of atomic weight, even though atomic mass is technically the correct term. Both are frequently assigned the unit of grams. The two properties, w(eight) and m(ass) are related through the mathematical function involving a (g)ravitational constant, by
w = g x m
The amount of substance is described by the mass, whereas weight is how it responds under gravity. Mass is constant, but weight varies according to how great the gravitational field is to which the mass is exposed. An apple would weigh more on Jupiter than it does on Earth, but it’s mass is the same.1
Particle Mass
All atoms are made of the same constituent particles: protons, neutrons, electrons. These particles differ in more than electrical charge. They differ also in mass.
Proton — 1.673 x 10-24 grams
Neutron — 1.675 x 10-24 grams
Electron — 9.109 x 10-28 grams
The neutron has nearly 2,000 times the mass of an electron, while the neutron and the proton are nearly equal in that regard. So the presence of added neutrons increases the mass of an atom considerably.
Neutron Variation in Isotopes
One might anticipate that, if so tiny an atom as helium can have as many as two extra neutrons and remain stable, surely larger atoms with considerably more protons should be able to vary by many more than two neutrons. Is this a valid supposition? Not really. Even very large atoms have a collection of stable isotopes represented by a single digit. Tin, alone, has as many as 10 stable isotopes. So percentages of variation in mass are greatest in the lightest elements, notably hydrogen and helium.
This is reflected in atom behavior, especially for the chemically somewhat reactive hydrogen atom. Reflecting how hydrogen atoms are considered in bulk, the hydrogen atom with two neutrons (deuterium) instead of one (protium) has been nicknamed “heavy hydrogen”. Deuterium is almost exactly twice as heavy as protium!
So it is that protium and deuterium may behave differently despite the fact they are basically the same in their overall chemistry. The kinetic isotope effect, descriptive in name, describes and quantifies how they differ in rates of reaction (notably where a transition state is encountered) based on the isotopes used. This is especially useful in elucidating the steps of an organic chemical reaction.
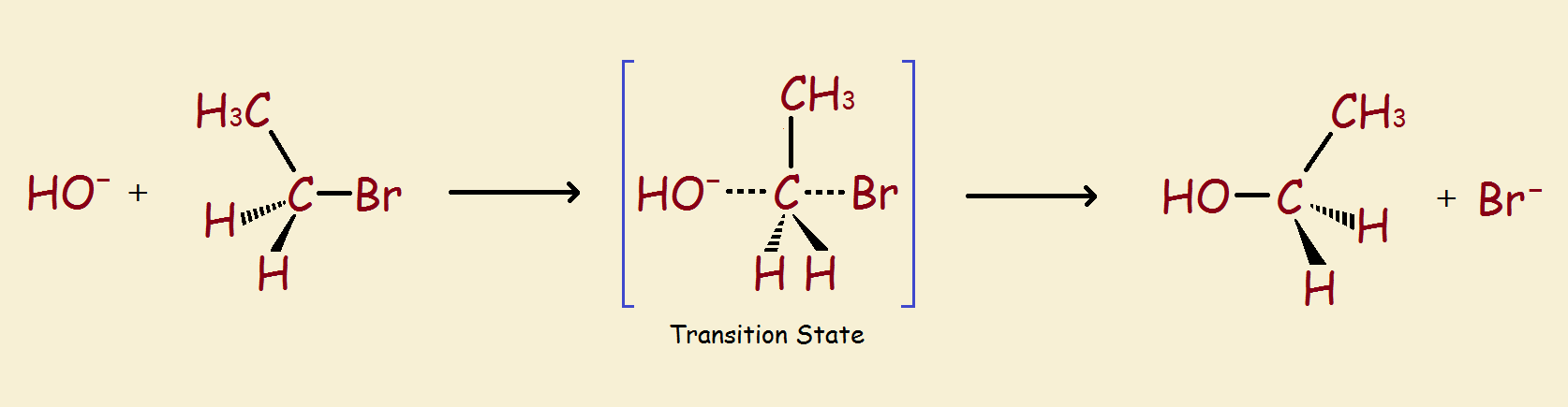
Kinetic Isotope Effect
Thus one online definition of the isotope effect succinctly states, “The kinetic isotope effect… is the change in the… rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes.”
1 Gravity is of little importance in the world of the atom. See, How Important is Gravity in the Atom?
Note: You might also enjoy What Happens at Absolute Zero?
References:

So, are you saying that the chemical reaction above, in which a bromine ion is replaced by a hydroxyl ion takes place at a different rate if any of the hydrogen atoms (whether single or as hydroxy?) are deuterium, rather than protium?