
Metals are electrically conductive, yes. But is there such a thing as a polyacetylene conductive polymer? In a sense, a plastic that is not electrically insulative, but electrically conductive, and a top-notch conductor at that?
Ethyne (AKA acetylene) polymerizes to polyethyne (polyacetylene) in the presence of copper catalysts. When prepared in an alternate way, polyacetylene (CH)2 exhibits moderate electric conductivity. Oxidation with iodine increases conductivity of the polymer by several powers of ten. In fact, it approaches the specific electrical resistance of silver. In 2000, Shirakawa, Heeger and McDiarmid received the Nobel Price for this discovery.
How is the reaction written? And what is so special about polyacetylene conductive polymer?
General Schematic Preparation of Polyacetylene
The simple saturated molecule ethane is written H3C-CH3. Remove two molecules of hydrogen from this structure, and you get acetylene. Acetylene’s structure is written H-C≡C-H. This can be reacted to form polyacetylene.
n H3C−CH3 → [−HC=CH−]n + 2n H2↑
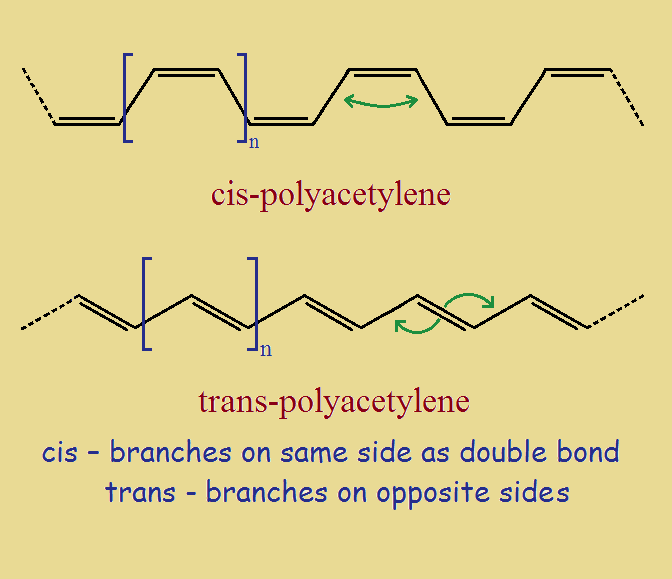
Cis- and Trans- Polyacetylene
Although there are a variety of forms polyacetylene can take, linear chains are of greatest interest. The two linear varieties are the cis- and trans- polyaceteylenes. Their ideal structures are shown in the image below. Hydrogen atoms (or substituents) are located at each vertex.
Conjugation refers to the alternating single bond, double bond, single bond, double bond, and so on.
Conductive Polymer
Conjugating the carbon bonds allows for an electric flow. This is because a double bond is the combination of a sigma bond and a pi bond. A sigma bond lies between carbon atoms, while a pi bond lies above and below the bond. A pi bond acts as if it is mobile. Visualize the transfer, below.
=C−C=C−C=C−C=C− → −C=C−C=C−C=C−C=
The pi bond on the first carbon flips to the second carbon atom. In turn, the double bond at the second carbon flips to the third carbon. And so the electron in the pi bond moves along the carbon skeleton. A flow of electrons is called an electric current.
Halogen Doping
Doping polyacetylene with iodine increases its electrical conductivity greatly. Under certain conditions, superconductivity has been achieved. No wonder the individuals most closely involved in this project won the Nobel Prize.
Why the Excitement?
Why all the hoopla in developing a polymer whose conductivity compares to that of a metal? Why not simply use a metal when good conductivity is needed? Besides, polyacetylene has serious drawbacks. For one thing, it lacks stability in air.
Well, the concepts behind this work are being applied to other polymeric organic compounds. In addition, polymers can be prepared and fabricated in special ways. They have suitable properties metals do not have. This makes them suitable for niche applications.
The following video covers the history of polyacetylene and helps us visualize what the excitement is all about:
Note: You might also enjoy: Acetylene and Acetylides
References:

The reader will no doubt realize that the hydrogen atoms on cis- and trans- polyacetylenes vary in relative locations. For trans-, the hydrogen atoms on double-bonded carbon atoms are on opposite sides of that bond. For cis- they are on the same side. Recall that trans means across, and cis means same.
[…] Polyacetylene Conductive Polymer – Quirky Science […]