 There are thousands of chemical compounds in nature. They are divvied up into categories or classifications. These include hormones, alkaloids, vitamins, neurotransmitters, enzymes, proteins, and nucleic acids. One group of chemicals follows a structure rule called the isoprene rule.
There are thousands of chemical compounds in nature. They are divvied up into categories or classifications. These include hormones, alkaloids, vitamins, neurotransmitters, enzymes, proteins, and nucleic acids. One group of chemicals follows a structure rule called the isoprene rule.
Many every day substances are part of this group. One example is turpentine. Another is beta-carotene (the source of the orange color in carrots). Chemicals such as these derived from isoprene are called terpenoids.
Structure and Statement of the Isoprene Rule
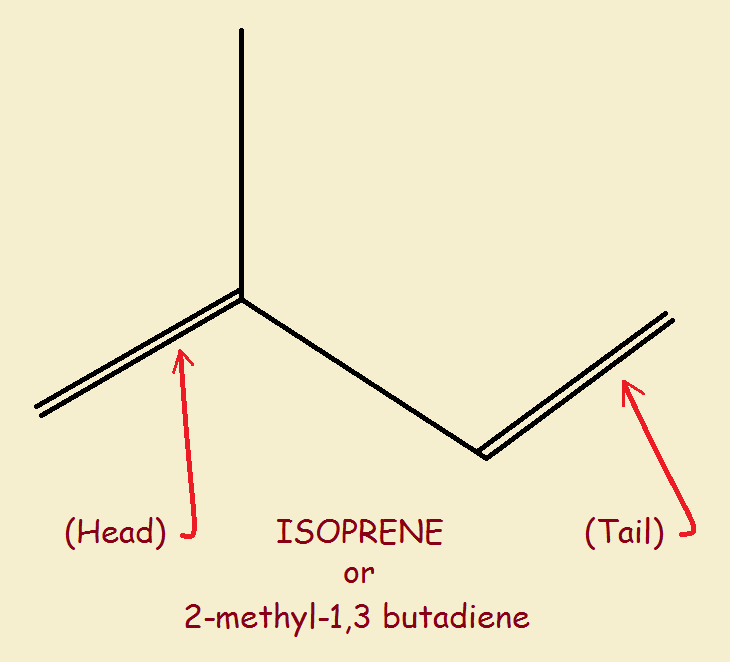
Isoprene consists of carbon and hydrogen. But it is not limited to single bonds. See the image of isoprene (C5H8) featured with this article. The double lines stand for double bonds. Since the carbon pairs involved have two bonds rather than one, a hydrogen atom is missing from each of them.
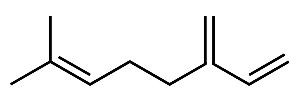
Now, isoprene has a head and a tail. The isoprene rule dictates how isoprene units are joined together in a head to tail configuration. The tail of the first molecule joins the head of the second molecule to form a larger unit. The simplest example is myrcene. This substance is used as an intermediate in the manufacture of flavor and fragrance chemicals.
Limonene is a terpenoid cleaning agent. It follows the isoprene rule. Like myrcene, limonene is formed of two isoprene units. However, they are joined in such a way as to form a ring structure.
As mentioned earlier, the chemical causing the carrot’s orange color is beta-carotene. Break this chemical in half, and you get retinal. Retinal is a form of Vitamin A. It is essential for good eyesight. Do you see why carrots are good for your eyes?

In Conclusion
There is a huge number of terpenoids. A few examples are pinene, geraniol, menthol, and camphor. Maybe one day you will study terpenoid chemistry. If so, I guarantee you will not be bored!
Note: You might also enjoy Organic Chemistry: Pushing Electrons
References:
