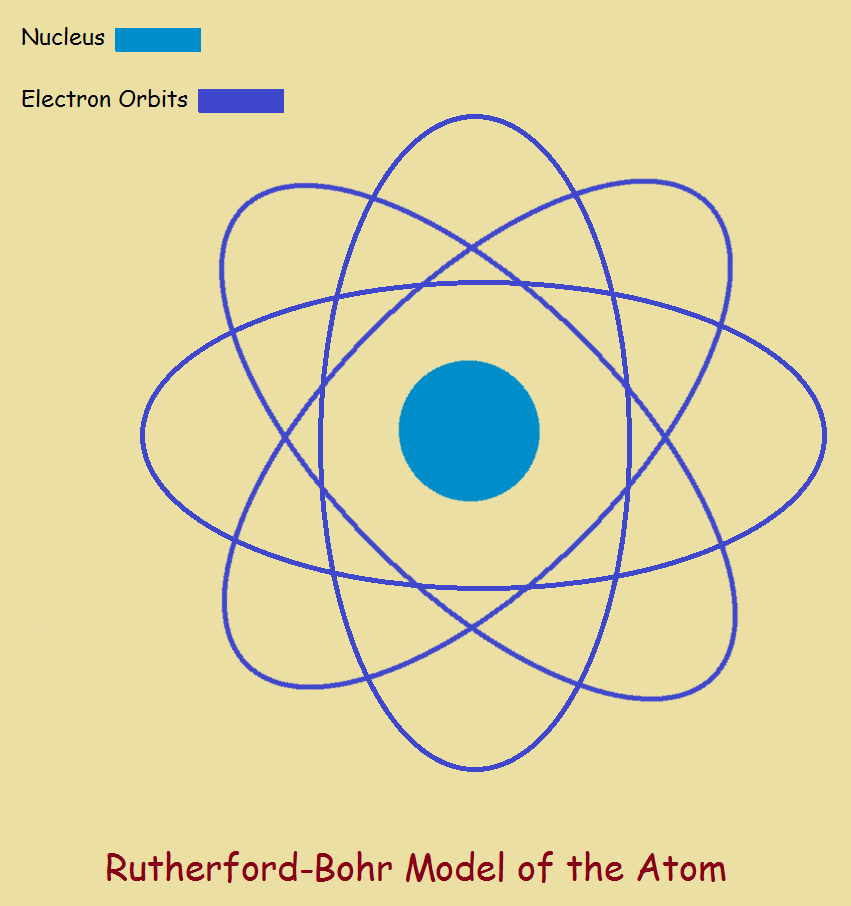
Atoms are constructed of a central nucleus, containing positively-charged protons and uncharged neutrons, plus orbiting, negatively-charged electrons, in number equal to the number of protons.
Although an electron carries a charge equivalent (though of opposite polarity) to that of a proton, its mass is a mere 1/1836th that of a proton.
Some mistakenly think the electron isn’t a particle at all, but a cloud. This inaccurate notion doubtless arises from the cloud-like appearance of the probability distribution curve of an electron in its orbit.
At any rate, an electron generally exhibits particle-like properties, and is best mentally envisioned as a particle.
How the particle we call an electron behaves depends upon the condition in which we find it. There are important differences between bound and unbound electrons.
The Free-Moving, Unbound Electron
Pull out a gun and shoot a bullet, and in many ways, an electron is like that bullet.
It has mass — it has momentum. One additional thing the electron has that a bullet does not have, is charge.
Consistent with classical behavior, an unbound electron can absorb energy in any quantity. Such is not the case with an electron bound as part of an atom or an atom fragment.
The Restricted, Bound Electron
Atoms as a whole can absorb energy in any amount, but the electrons that orbit the atoms or atom fragments cannot. They only absorb energy in specific or “discrete” quantities. The reality of this was first expressed mathematically, in the case of the simplest atom, hydrogen, by physicist Niels Bohr. He developed a mathematical expression that, in essence postulated such discrete behavior.
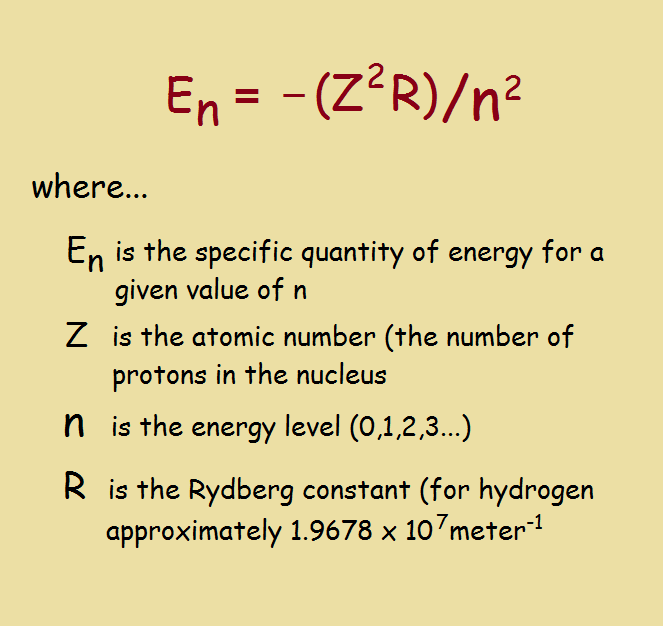
Plug in the values of Z, n, and R, where n must be an integer, and it quickly becomes apparent E cannot be just any value. Although scientists intended for this equation to explain the hydrogen atom, and it would be self-defeating to apply it to any other atom, the concept of discrete orbiting electron energies applies across the board.
What If Bound Electrons Could Absorb or Emit Energy in Any Amount?
If a bound electron could absorb or emit any amount of energy as it orbited, logically, it would continually lose energy consumed in the orbiting process. It would gradually spiral into the nucleus. If that were the case, the universe as we know it could not exist.
Now, consider a lone hydrogen atom placed in an environment where it receives exposure to a variety of energy sources…
If heat energy is applied, the atom as a whole will increase in kinetic energy, but the individual electrons go largely unaffected.
Now let us switch to discussing photons. Photons of different frequencies possess different, specific, quantities of energy.
It takes a photon of an acceptable quantity of energy, properly located, for an electron to absorb it. If that happens, the electron jumps to a higher energy level, an orbital where n>1. How large the jump is depends upon the amount of energy the photon contained. If n is higher than 2, it is possible at some point for the now unstable, energized electron, to lose some or all of its energy, in steps. The n-value decreases in steps. That is, the electron not only must gain energy in steps, it must lose energy in steps.
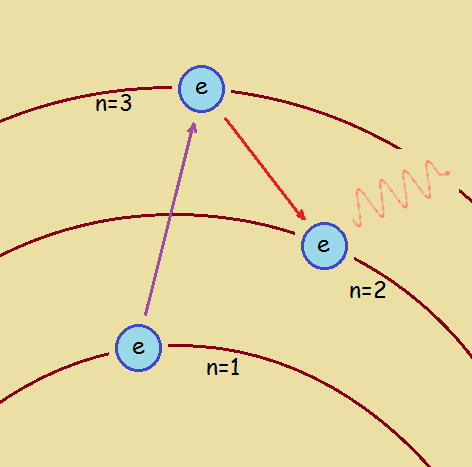
Bound and Unbound Electrons: Important Differences
Important differences, depend in part upon the creation of a new entity. In binding to an atom, the electron loses some of its personal unbound freedom. It is no longer “one flesh”. It cannot entirely act as if it were unbound. It can absorb and emit only certain specific amounts of energy, called quanta. There are important differences between bound and unbound electrons.
These quanta are associated with photons. Some photons (those in the visible range of the electromagnetic spectrum) are associated with color. Bound electrons are essential in the colors of nature. Bound electrons also exhibit various behaviors in magnetic fields. Such interactions are important in analytical chemistry and in the field of medicine.
Note: You might also enjoy The Neutron A Stable Particle?
References:
- Nave, Carl R. Department of Physics and Astronomy. (2000). Georgia State University
- University Of Nebraska-Lincoln. Astronomy Education: Transitions
← Back to Math-Logic-Design
← Home

Do you find unbound electrons available on earth or are they only in space? Or are these used in chemical or physics experiments? Maybe Lasers? Is that why lasers are so powerful?
Lasers are light. Free electrons are everywhere.