 Atoms and molecules are small indeed. Until recently, catching even a glimpse an atom was impossible. It still is impossible to see a chemical bond. Despite that, we know quite a few chemical reactions and can predict how many more will turn out. But we could know ever so much more about the scientific world of the very small if we had a very close bond model.
Atoms and molecules are small indeed. Until recently, catching even a glimpse an atom was impossible. It still is impossible to see a chemical bond. Despite that, we know quite a few chemical reactions and can predict how many more will turn out. But we could know ever so much more about the scientific world of the very small if we had a very close bond model.
We will discuss three bond models that have been used in the past, and to some extent still are used.
- The rigid model
- The spring model
- The force / charge model
See the images associated with article. Each depicts one of the models discussed below.
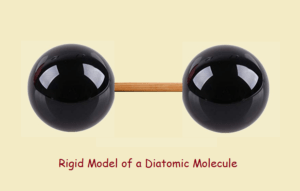
The Rigid Model
One can depict a diatomic molecule by joining two balls with a thin dowel rod. Under such a model, what deductions might we make concerning the rod (the chemical bond)?
We might conclude that in a molecule, the bond maintains a constant length. It does not bend. It does not allow the balls any freedom to spin, individually, on the dowel. In fact, the atoms can move about in 3D space, but only as a rigid unit.
The Spring Model
The spring model allows increased freedom, for the bond and the atoms. The spring (bond) can expand and contract. It can bend.
The atoms (balls) can be twisted some, relative to each other. The atoms do not have freedom to spins individually on the dowel, however.
The Force / Charge Model
It is not truly possible to depict a force / charge, visually. The image provided shows two atoms connected by the usual iron-filing depiction of magnetism. We must let the mind wander a bit, visualizing “what gives” in this scenario.
The bond distance could vary, simply by applying an additional pulling or a pushing force to the atoms. Likewise, the molecule would be flexible to bending. Twisting, indeed rotation, should be possible for the atoms.
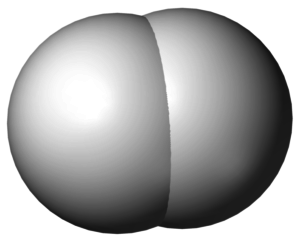
The Space Fill Model
There is one useful molecular model we will not discuss here, except in passing. It is useful to the chemist, but does little to help us understand the nature of the chemical bond. The topmost image of this article illustrates the diatomic hydrogen molecule.
How Does a Diatomic Molecule Actually Behave?
For purposes of discussion, we’ll choose the simplest diatomic molecule, hydrogen H₂. What freedom of movement do diatomic molecules such as hydrogen exhibit?
The bond between atoms can both increase and decrease in length. In fact, given the correct circumstances, it can oscillate back and forth. This stretching is the behavior typical of a linear harmonic oscillator. That is, the behavior of an ideal spring.
This effectively eliminates our rigid model of the atom.
Spectroscopy reveals that bending of the bond and rotation of the atoms is also characteristic of molecules. Thus the spring model is not entirely correct.
The force / charge model alone matches up with the behavior of atoms. Yet, the other two models serve a purpose, yes, even the rigid model. How so?
The Disqualified Models are Still Useful
It is a difficult thing to visualize in 3D. The chemist, particularly the organic chemist, needs something he can see, something he can hold, to best visualize complex molecules and the reactions they undergo.
The slight improvement in sophistication of the spring model is unnecessary in most instances, and can be awkward to use. Hence ordinary chemical model kits consist of balls and bonds, the bonds sometimes being made of slightly flexible rubber or plastic.
The author himself possesses the same two molecular model kits he purchased when he attended University some 50 years ago!
Note: You might also enjoy Which are Stronger? Covalent or Ionic Bonds?
References:



So even models that are incorrect can serve a purpose!
In a sense. Or, perhaps, a little less than completely correct.