“And the leaves of the trees were for the healing of the nations.” -Revelation 22:2c
While the above quote is not intended as a discussion of home remedies, people have converted roots, leaves, flowers, and stems to teas, emoluments, and powders for the purpose of healing for many, many years.
Of greater interest to us, you can use flowers and spices in much the same way a chemist uses commercial indicators in chemical titrations in the laboratory.
A titration is the measured addition of a solution of known concentration into an another solution of unknown concentration with which it reacts, with the goal of reaching a proper end-point. And how does one know when the end-point has been reached? By means of an indicator. One form of indication is through a color change when the point is reached.
Acid-Base Titration
Acids and bases react chemically to produce near-neutral compounds called salts, of which table salt is an excellent example. Combine a hydroxide base with an acid in the correct quantities, and you get the corresponding salt and water.
M-OH + H-A → M-A + H2O
Now this sort of reaction is of great importance. The difficulty is in determining when one combines the correct amount of M-OH and H-A to give the salt and water (notice the number of M atoms, H atoms, and O atoms on the left side of the reaction arrow equal the number on its right side).
If one combines the correct amounts of M-OH and H-A by weight, only pure M-A and H2O remain after the reaction. If one uses too much M-OH, the result is
M-OH + H-A → M-A + H2O + (remaining) M-OH
If one uses too much acid, the result is
M-OH + H-A → M-A + H2O + (remaining) H-A
Chemists usually want the reaction quantities to be correct for complete conversion.
In order to accomplish this, they often add a very small quantity of a substance called an indicator. An indicator tells the chemist when to stop adding the second chemical, whether the second chemical is the acid or the base. Frequently, the indication is a change in color. Most often, the indicator changes from one color to another or from colorless to a color.
Commercial Indicators – Litmus
Chemical supply houses carry many different chemical indicators. The most familiar of these is litmus. Litmus is available as paper strips that one dips in the solution being titrated. It turns blue in base and red in acid. If one inserts blue litmus paper into a beaker of base solution and adds drops of acid, with swirling, the blue paper will remain blue until adding one drop of excess acid solution. At that point, the litmus paper will begin turning red. The reaction is complete.
The Fun Begins – Flowers
Whether you’re just interested in science, or you’re a home school parent, you can use materials found in nature or the grocery store to demonstrate the principle of acid-base titration. People can also make natural indicators from flowers.
In the case of flowers, petals may be crushed and the resultant liquid whether alone or mixed with a bit of added alcohol or water to an acid such as vinegar and a base such as sodium bicarbonate (which is much safer than sodium hydroxide).
In this instance, the reaction becomes,
CH3-COOH + NaHCO3 → CH3-COONa + H2O + CO2↑
“Translated” into English, this reads: acetic acid plus baking soda yields sodium acetate, water, and carbon dioxide gas, which causes no problem because it escapes into the air.
Trying out various flowers will demonstrate which ones are best as indicators. Generally, the greater the color change, the better an indicator is, since observers can see the change more quickly. Darker flowers may work best.
The Fun Continues – Turmeric
As mentioned earlier, some spices can be used as indicators. Outstanding among these is turmeric. Turmeric is a lovely yellow spice derived from the rhizome of Curcuma Longa. It is a feature of Asian cooking but is also eaten in Africa, South America, and many other lands.
To understand how turmeric can serve as a chemical indicator, it is vital to discuss two technical topics: keto-enol tautomerism and the concept of chromophores.
Keto-Enol Tautomerism
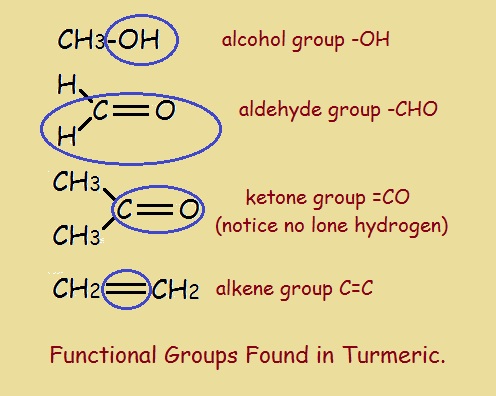
Carbon-based organic compounds are put into categories according to functional groups. For instance, alcohols have an –OH group attached to at least one carbon atom. Carbonyl compounds (aldehydes and ketones) have at least one carbonyl group –C=O. Alkenes possess a double bond between two adjacent carbon atoms instead of a single bond.
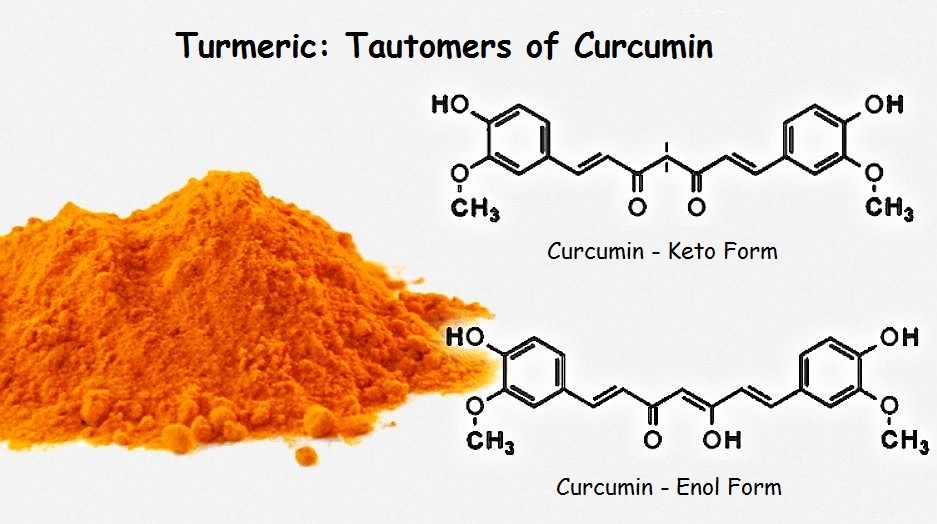
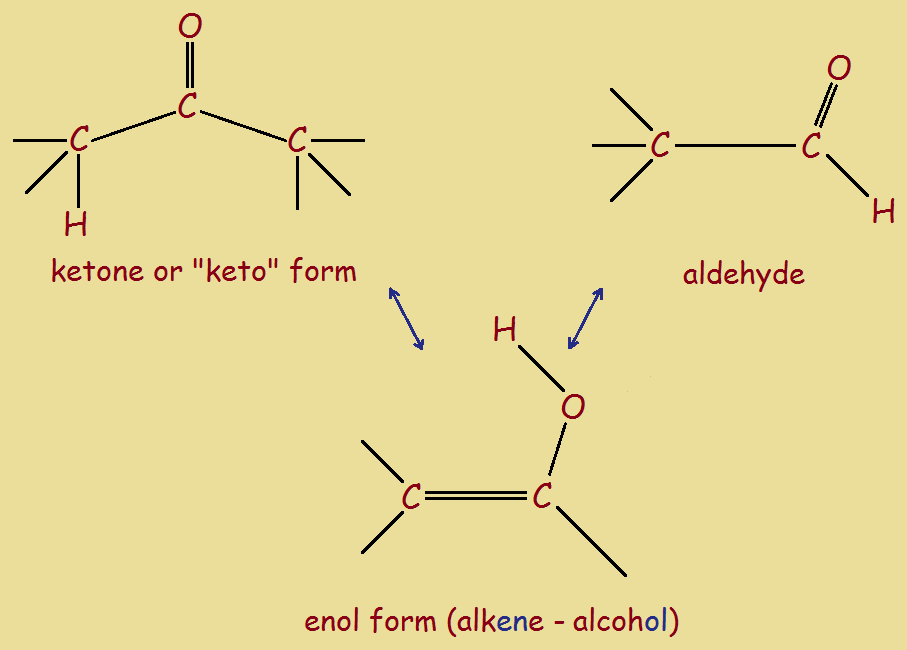
Under certain relatively minor conditions, ketones may in some instances adopt an alkene-alcohol configuration (or enol for short). This is an equilibrium change. That is, the enol form can easily return to the keto form. For turmeric, the transition is readily triggered by adjustment of the acidity and alkalinity, or pH. Actually, it is not the turmeric in its entirety that undergoes the change, but the chemical compound curcumin.
Curcumin has two ketone carbonyl groups, but only one can convert to an enol form. Notice the little red markers drawn in the keto form for curcumin. The double bonds in the enol form alternate throughout the complete molecule, but the markers show that such is not the case for the keto form.
Chains of double bonds constitute part of a chromophore. It is necessary to establish just what a chromophore is in order to understand why turmeric makes a suitable acid-base indicator.
Understanding Chromophores
Simply defined, a chromophore is the portion of a molecule that absorbs particular frequencies of light, reflecting the remainder of the light, which, because it no longer represents the complete or white spectrum, bears color.
If the chromophore absorbs yellow, the substance appears blue; if it absorbs red, the substance appears green.
The length of a chromophore has a lot to do with its color. The enol form of curcumin has a chromophore about twice the length of the keto form. The presence of base favors the enol form, and leads to the formation of a reddish color; whereas the keto form is faintly yellow. Clearly, the longer chromophore favors the lower frequency red, in contrast to shorter chromophore of the keto form favoring the higher frequency yellow.
Demonstration of Turmeric as Chemical Indicator
Turmeric thus changes from nearly colorless to red as pH increases or is made more basic. This short YouTube video aptly demonstrates the use of turmeric as a chemical indicator.
Spices, Flowers, and Chemistry
You may wish to try various flowers and other spices to see if any of these have the ability to serve as chemical indicators. If so, it should be interesting to learn if keto-enol tautomerism is again at work, or if there is some other mechanism that explains the chromophoric reaction.
Note: You might also enjoy Make Homemade Iron Gall Ink from Oak Tree Galls
References:
- Oregon Museum of Science and Industry (OMSI): ChemLab Take Home Activities. (2007)
- Stankovic, Ivan: Curcumin. (2004). Food and Agricultural Organization of the United Nations (FAO)

That’s a very useful and educational article. I love turmeric and even like it as part of a drink, eg ginger and turmeric tea.