
One of our readers asks, “So, is the crystallization process of cane juice to raw sugar considered a chemical change or a physical change? Why one or the other?”
Let’s discuss, starting with an explanation of what constitutes a chemical change.
What is a Chemical Change?
A chemical compound consists of atoms bonded together in specific fashion to form molecules. When you modify the combination of atoms, you’ve achieved a chemical change.
For instance, combine sodium hydroxide with hydrochloric acid, and the result is sodium chloride (table salt) and water, according to the reaction:
NaOH + HCl → NaCl + H₂O
This example clearly represents a chemical change. Salt (NaCl) is edible, sodium hydroxide or lye (NaOH) is not. Water (H₂O) is safe for human consumption, hydrochloric acid (HCl) is not.
Physical Change
An example of a simple physical change is the freezing of water into ice, as it goes from a liquid to a solid state. No real change in chemistry has taken place during the freezing process, which is completely reversible. On the other hand, most chemical changes are not readily reversible.
When Change is Not Black and White
Sometimes what appears to be a physical change might also be a chemical change. Take polymers. Polymers are long chain molecules—sometimes made into cloth. If you take a sheet of polymer cloth and cut it with scissors, actual molecules are cut. Two shorter and lighter polymer molecules are produced.
Then, too, a chemical change may also produce a physical change. Consider the chemical reaction between sodium sulfate solution and calcium chloride solution. Ignoring the water solvent, we write the equation,
Na2SO₄ + CaCl2 → 2 NaCl + CaSO4↓
How does this suggest a physical change occurs? The down arrow next to the calcium sulfate indicates it does not remain in solution, but precipitates out as a finely divided solid.
Cane Juice Crystallization Yields Raw Sugar

Sugarcane is a tropical grass. It has been known and grown for thousands of years. Crushed, the sugarcane stem produces juice that yields the lion’s share of the world’s table sugar, sucrose.
But cane juice is not a simple combination of sucrose in water. Cane juice crystallization yields raw sugar, which must then be refined to yield the white stuff.
Commercial processing features crushing the sugarcane stalks so they release their juice. The crushed canes yield more if they are repeatedly treated with a little hot water and crushed again.
To this juice is added a little lime (CaO) and some carbon dioxide gas to prevent the sucrose from converting into other forms of sugar that do not crystallize well. Next, water is boiled off, the last of it under vacuum, crystallizing out the raw sugar. The crystals are gathered by centrifuge and dried. The resulting raw sugar contains needs refining to remove impurities.
You may enjoy the following video on the juicing operation…
At the Refinery
Further processing involves physical processes, such as dissolution, filtration, and recrystallization.
So the answer to our title question is – The change is physical.
Yet, we can gain further insight into the subtleties of both physical and chemical change.
Examination from Another Perspective
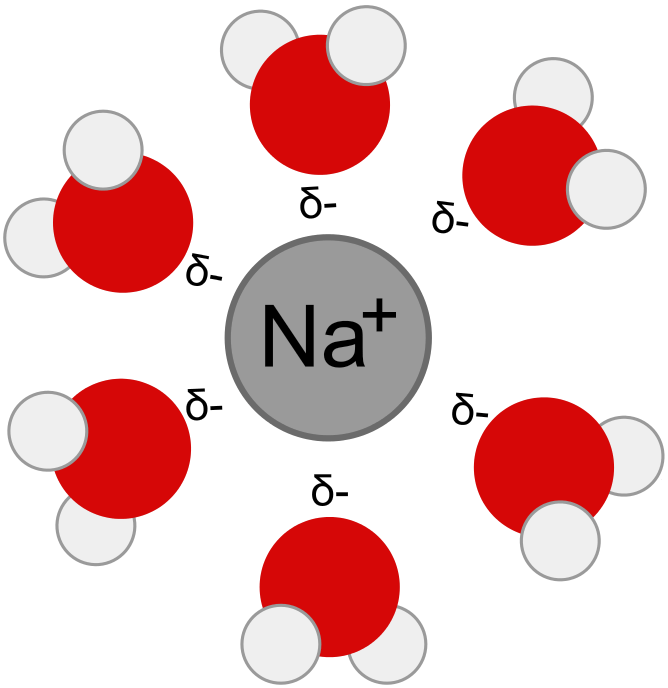
If we avoid a black and white perspective, we see that a reaction can be partly physical and partly chemical. The percentages vary greatly.
Consider dissolving table salt in water. Ordinarily, this is considered a 100% physical reaction, since on evaporating the water, we regain the original quantity of sodium chloride.
Table salt molecules are ionized almost completely upon dissolving in water. We write,
NaCl → Na⁺ + Cl⁻
In reality, the charged sodium atoms (ions) are surrounded by molecules of water. The partial negative charge of the oxygen atom in a water molecule is attracted to the positive sodium ion. Weakly yet importantly, the water molecule bonds to the ion. In fact, a number of water molecules bond to each sodium ion. Scientists call these important bonds hydrogen bonds.
Similarly, the partial positive charge of a hydrogen atom in a water molecule is drawn to the negative chlorine ion, forming a weak bond to it. Again, multiple water molecules do so.
Logically, one could argue that a chemical change has taken place. The chemical reaction, they might suggest, should read,
NaCl + (x+y)H2O → [x(H2O)Na]⁺ + [y(H2O)Cl]⁻
Depending upon the concentration and other factors, both x and y values may be up to six.
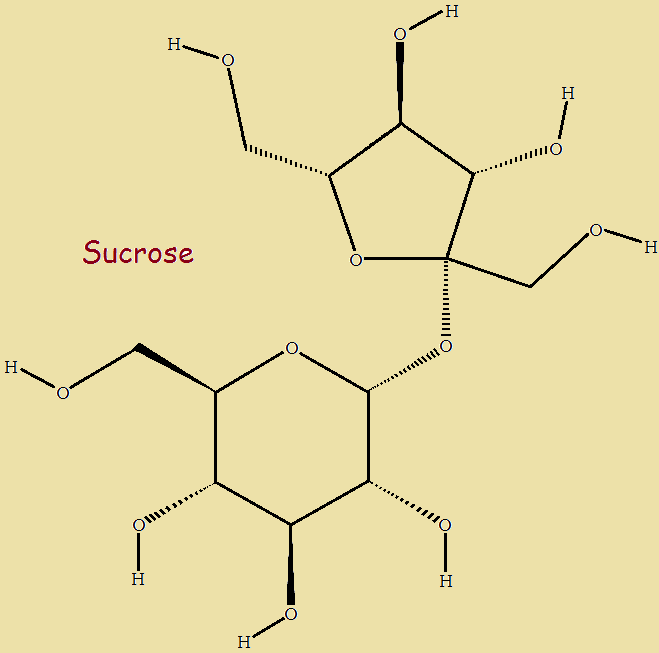
This graphic illustrates the chemical structure of sucrose. Copyright image by Vincent Summers, all rights reserved.
How Does This Relate to Sugar?
When sugar molecules crystallize they come into close proximity, one to the other. As water boils away, the sugar molecules establish hydrogen bonds with each other.
They do this because the crystal contains less energy than loose, random molecules do. Less energy means greater stability.
Thus cane juice crystallization, if one really presses the issue, could be considered a kind of chemical reaction. Still, for all practical purposes, the procedure constitutes a physical, not a chemical reaction.
Note: You might also enjoy Cooked Blueberries Taste Different Than Raw – Why?
References:
- Agricultural Marketing Research Center. Sugarcane Profile. (2017)
- Baucum, L. E, et al.: An Overview of Florida Sugarcane. (1992). University of Florida – IFAS Extension
- The Sugar Association: Refining and Processing Sugar
- Brown, George: Sucrose – Precise Determination of Crystal and Molecular Structure by Neutron Diffraction. (1963). American Association for the Advancement of Science
- Husband, Tom. The Sweet Science of Candy Making. (2014). American Chemical Society
← Back to Food and Health
← Home
