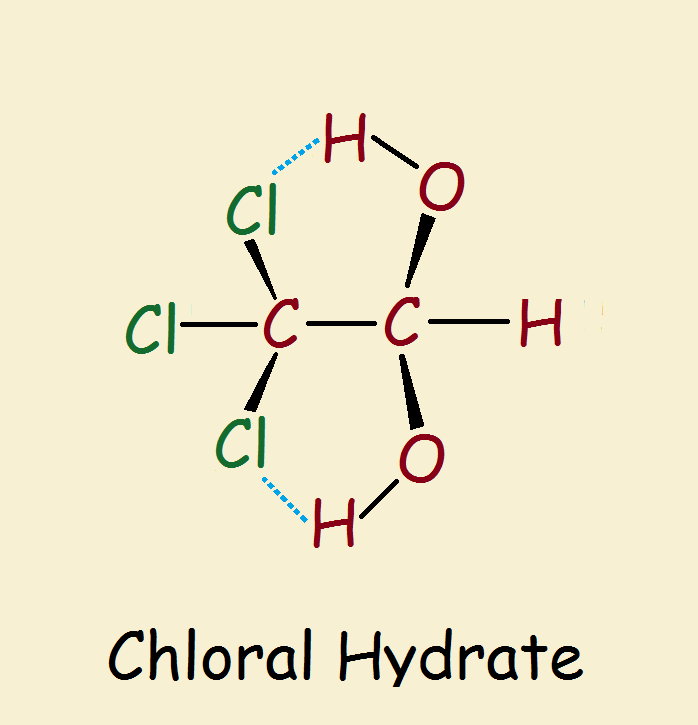
 Among the endless thousands of organic compounds that are familiar to the public, one is associated with foul bars, booze, and foul play. It is chloral hydrate (Cl₃C-CH(OH)₂). Combined with an alcoholic beverage, the result, an infamous “Mickey Finn” causes the victim to pass out as quickly as if he had been knocked out.
Among the endless thousands of organic compounds that are familiar to the public, one is associated with foul bars, booze, and foul play. It is chloral hydrate (Cl₃C-CH(OH)₂). Combined with an alcoholic beverage, the result, an infamous “Mickey Finn” causes the victim to pass out as quickly as if he had been knocked out.
Mickey (Not Steamboat Mickey) In the Movies
In old-movie and TV shows, the bad guy (often in a saloon) wants to capture his victim by rendering him unconscious. Maybe he even wants to sell the man to a ship’s captain who needs to complete his crew. The poor guy is Shanghaied! An interesting example is this old Bonanza western TV show episode:
Was There an Actual Mickey Finn?
It appears there was! In the latter part of the 19th century into the early 20th century in Chicago, there was a Mickey Finn who owned a saloon. To his “honor” the expression “Slip him a Mickey” is attributed. See the reference, below.
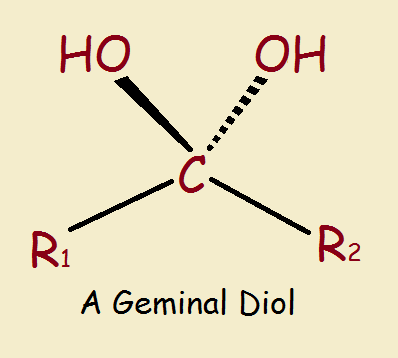
Chloral Hydrate: a Geminal Diol
An alcohol as the general formula R–OH, where R is some non-aromatic alkyl structure, and –OH is the hydroxyl group, consisting of an oxygen and a hydrogen atom. The word diol means there are two of these –OH groups, and the word geminal (“twins”Latin, gemini) means they are attached to the same carbon atom.
Chloral hydrate is a geminal diol. So why the word hydrate? Hydrate refers to water. Chloral is a nickname for trichloroacetaldehyde (Cl₃C-CHO). Yes, a geminal diol is equivalent to a hydrated aldehyde or ketone.
The Issue of Stability
As might be imagined, two hydroxyl groups on the same carbon is not an especially stable arrangement. Loss of water is to be expected. Yet chloral hydrate is a relatively stable compound. How can that be?
There is one factor, and likely two, that favor its stability. The three chlorine atoms exert an electron withdrawing effect. This helps the two oxygen atoms to remain in place, one of them not being forced to depart.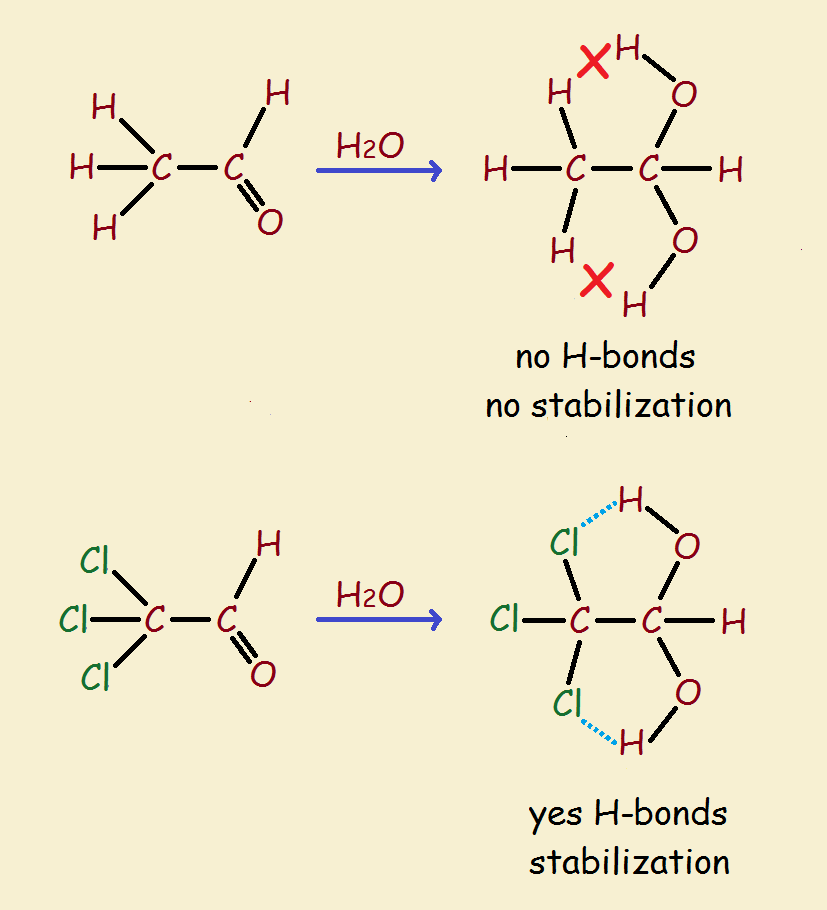
The More Speculative Stabilizing Factor
So what is the other factor? It is believed (and doesn’t it make sense?) that hydrogen bonding between the two hydroxyl hydrogen atoms and the chlorine atoms locks the water in place. In addition, such bonding produces structures are not unlike particularly stable, flat 5-membered rings.
In Conclusion
Chloral hydrate’s shady past doesn’t mean there are absolutely no beneficial uses of the substance. Neither is the administering of Mickey Finns the only bad use, however. Did you know that chloral (not the hydrate) was once used in the manufacture of the dreaded pesticide DDT?
This should not come as a total surprise, since the molecule is high in chlorine content. Highly chlorinated compounds are often used for their pesticide, bactericide, and fire retardant properties. Knowing this, does it make you want to run out and purchase chloral hydrate so you can consume some for its hypnotic properties?
References:
← Back to Food and Health
← Home
