 Notice the links in a gold chain, how they are not connected, but are a series of interlocked rings. There is a word that describes such an unconnected chain: concatenate. From this word, we can gain an understanding of what chemists call a catenane.
Notice the links in a gold chain, how they are not connected, but are a series of interlocked rings. There is a word that describes such an unconnected chain: concatenate. From this word, we can gain an understanding of what chemists call a catenane.
Now we are probably already familiar with typical multi-ring compounds, such as naphthalene. But naphthalene consists of two hexagonal rings sharing two carbon atoms, and joined together by them. Hence, naphthalene is not a 12-carbon structure, but a 10-carbon structure, C₁₀H₈.
Notice the simple illustration of one ring linking to another. This looks simple to achieve, but it is not so easy! We will not discuss the chemistry involved in preparing a catenane, but we will discuss some of the issues.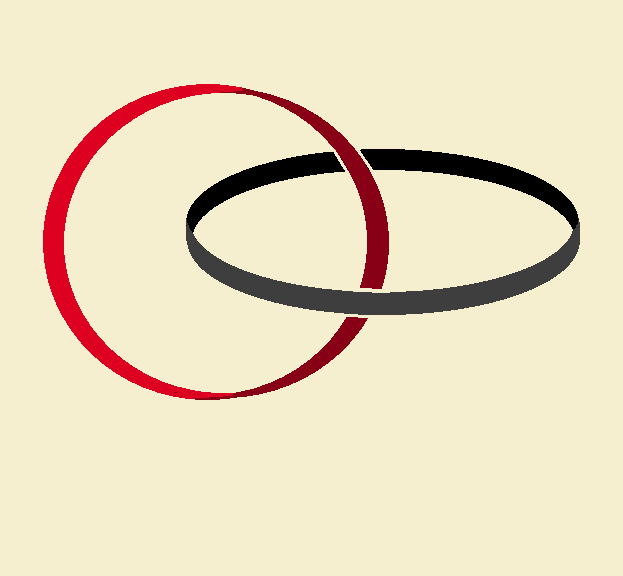
Why Not Simple Ring Closure?
It is a relatively simple matter to close up a linear molecule to produce a ring structure, rather like snapping together a string of beads. For instance, 5-aminohexanoic acid can be turned into δ-lactam (see the image) through the simple loss of one molecule of water.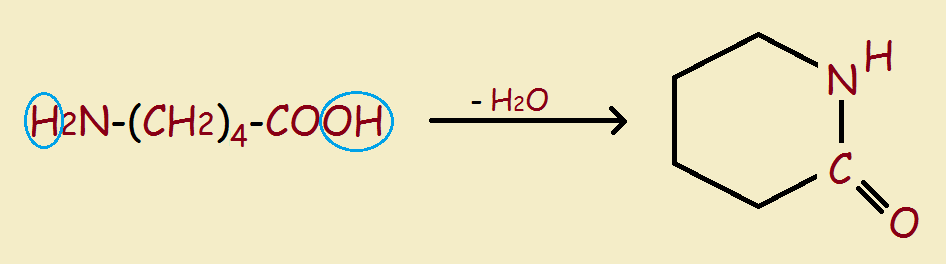
However, once a ring is constructed, why would another linear molecule want to pass partly through the center of the ring, then close to complete a two-link chain? Surely molecular forces would normally disfavor such an action. And they do!
In fact, the smallest, simplest ring molecule that can form a catenane from two of itself is cyclodecapentane, [C₁₅H₃₀]₂.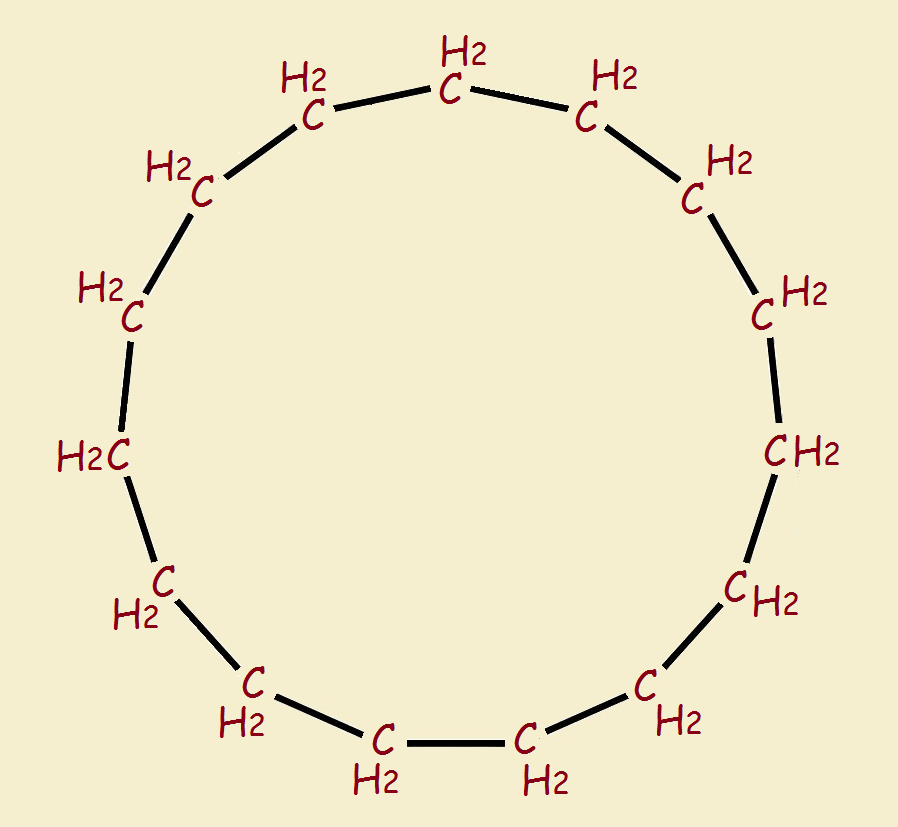
Cyclodecapentane
Sophisticated molecular mechanics programs point to the smallest ring that can unite with itself to form a two-link catenane as cyclodecapentane (see reference). Why is it apparently not possible to form a catenane from anything smaller? Consider the images that follow of the much smaller ring structure cyclohexane, C₆H₁₂.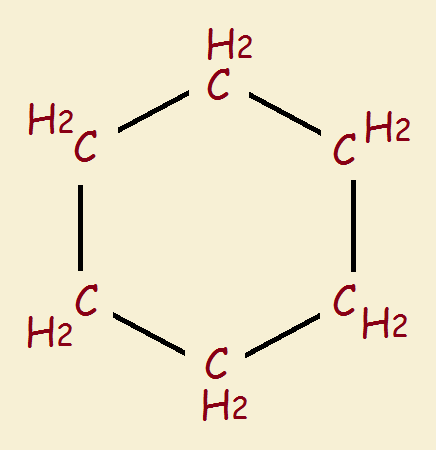
Written in simplest “stick” form we see a hexagonal ring that looks like there is ample space within the hexagon through which to pass a linear molecule, right? Well, a more accurate depiction is the “spacefill” model, which suggests strong electron repulsion would block passage. Not much of a gap, is there?
Spacefill would take a lot of that open space away from the cyclodecapentane ring, but enough would remain – or so calculations suggest – to allow the other ring, resulting in our simplest catenane.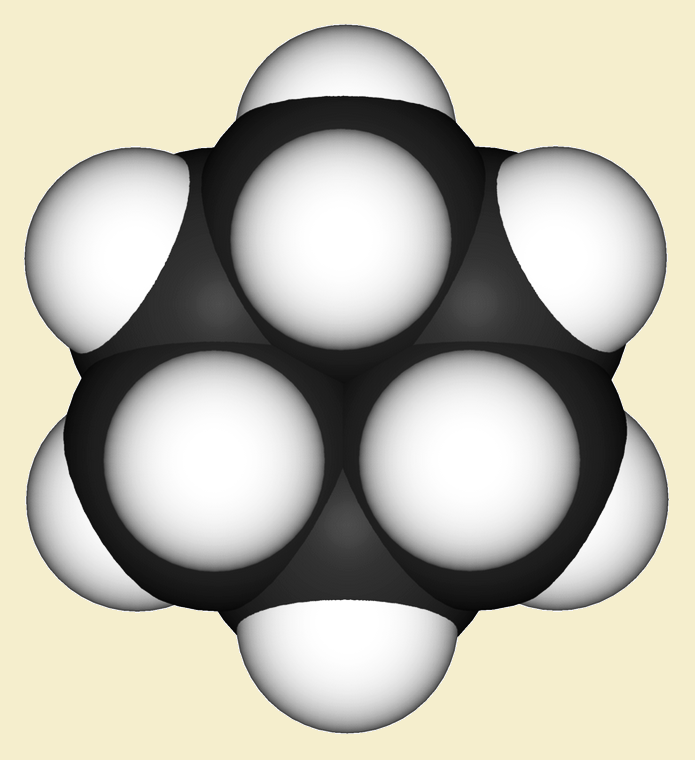
In Conclusion
There is ever so much more to catenane, rotaxane, and related structures than we can discuss here. Just one example of some of the fascinating research is seen in this University of Manchester webpage, entitled Hybrid Organic-Inorganic Rotaxanes.
References:
- Journal of Chemical Theory and Computation: How Small Can a Catenane Be?
- Org-Chem.org: Catenanes: The Art of Molecular Entanglement

Once again, you have found an interesting subject that I have never previously heard of!