Diatomic molecules have three translational degrees of freedom – but they have rotational and vibrational varieties as well.
How do all these degrees of freedom relate to the distribution of molecular energy?
To Begin With
The location of any particle lies within three-dimensional space.
The direction in which a particle moves is described by the three variables, usually written X, Y, and Z. As Ken Koehler of the University of Cincinnati informs us, atoms may be viewed as single points without size – so there are only three translational degrees of freedom for a given atom.
Degree of Freedom of Diatomic Molecules
Although it’s tempting to assume only three degrees of freedom exist for all “particles,” such is not the case for even so simple a particle as diatomic hydrogen gas.
Consider how diatomic hydrogen adds two more varieties of freedom, rotational and vibrational.
Rotational Degrees of Freedom
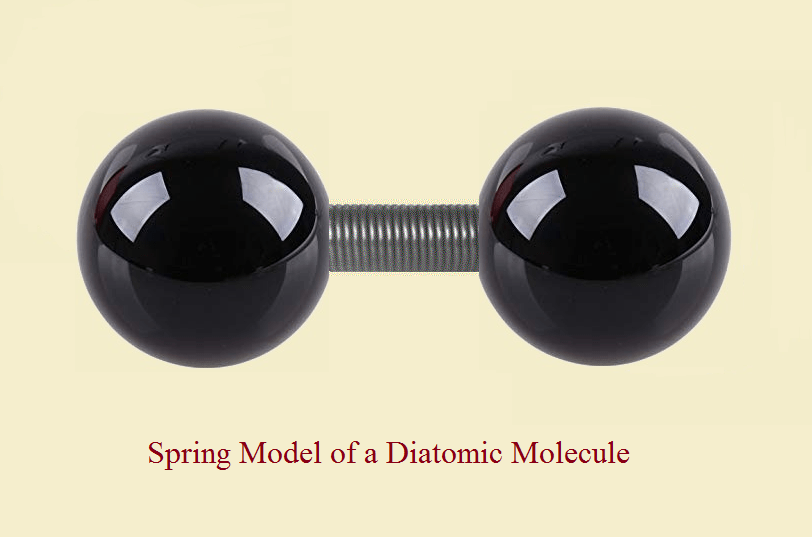
The standard model for diatomic molecules portrays them like a dumbbell with a stiff spring – this represents the atoms with the chemical bond in between the atoms. The translational motion of these molecules can still be described by three degrees of freedom.
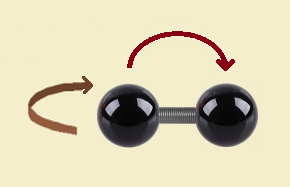
We must acknowledge, however, that the “dumbbell” can rotate in space as it travels, adding two more degrees.
Holding a pencil horizontally and visualizing two ways the pencil can rotate enables one to see rotation can occur in a clockwise or counterclockwise vertical manner, or in a clockwise or counterclockwise manner horizontally.¹
Vibrational Degrees of Freedom
But what are vibrational degrees of freedom? How may we visualize them? Since the bond resembles a rigid spring, at least to a reasonable degree, that “spring” can be stretched or compressed along the axis of the spring (or bond). By visualizing rapid stretching-compressing-stretching-compressing, the reader can understand why this form of freedom is termed “vibrational.”
Why do we say, “these degrees” – plural – rather than “this degree” – singular? Because if one atom vibrates in one direction, the other atom can either vibrate in the same direction, or in the opposite direction. In our spring model, the spring may be rigid, yet it can slightly stretch and contract.
And so, the total degrees of freedom describing the motion of a diatomic molecule is not three, for translation only, but seven: two degrees for rotation and two for vibration (some sources, e.g. Charles Kittel, cite only one vibratory degree of freedom kinetically, but include an equivalent amount of energy said to represent a potential energy contribution).
Degrees of Freedom are Both a Visual and a Quantitative Tool
Knowledge of degrees of freedom imparts better understanding of atomic processes.
If, for example, the temperature of hydrogen gas is increased, this means there is a corresponding increase of motion of the molecules. Distribution of the energy producing that motion occurs not only to the three translational degrees of freedom, making the molecules move through space more rapidly, but also to the two rotational and two vibrational ones, factors that might not otherwise have been visualized.
Since, as Georgia State University points out, equipartition of energy requires each degree of freedom receives an equal portion of the energy, three-sevenths of the energy goes into translation, two-sevenths into rotation, and two-sevenths into vibration.
We should add that the molecular freedom and their visualization are more complicated, the larger the molecular species becomes. However, the concepts involved remain the same.
¹ Why are there two and not three rotational degrees of freedom? Because it would require extreme energy to achieve the third rotation… so much so that it can be ignored. See the Physics Stack Exchange reference, listed below, for details.
Note: You might also enjoy Three Hydrogen Isotopes: Protium, Deuterium, Tritium
References:
- Takada, Kenjiro. Molecular Motions and Heat Capacities. (2004). Kyushu University
- University of Manchester. Molecular Spectroscopy and Mass Spectrometry
- Kittel, C. “Elementary Statistical Physics” Chapter 17: Thermodynamic Properties of Diatomic Molecules, by Charles Kittel. (1967). Dover
- Georgia State University – Hyperphysics: Equipartition of Energy. Equipartition of Energy
- Physics Stack Exchange: In counting degrees of freedom of a linear molecule, why is rotation about the axis not counted?
← Back to Classic Science
← Home
