 One tool of the organic chemist is that of oxidation. There are abundant reagents, which vary in reactivity and strength, to choose from to create this reaction. A plethora of techniques guarantee specificity. All are important to the synthetic organic chemist and to industry.
One tool of the organic chemist is that of oxidation. There are abundant reagents, which vary in reactivity and strength, to choose from to create this reaction. A plethora of techniques guarantee specificity. All are important to the synthetic organic chemist and to industry.
What is a reagent? It is a chemical that is of interest because it can react with the substance chemists wish to modify and study. Oxidizers are one kind of reagent.
Consider the progressive oxidation of methane (CH₄) from its lowest to its highest oxidation state.
The progression of the oxidation of methane products is written simply,
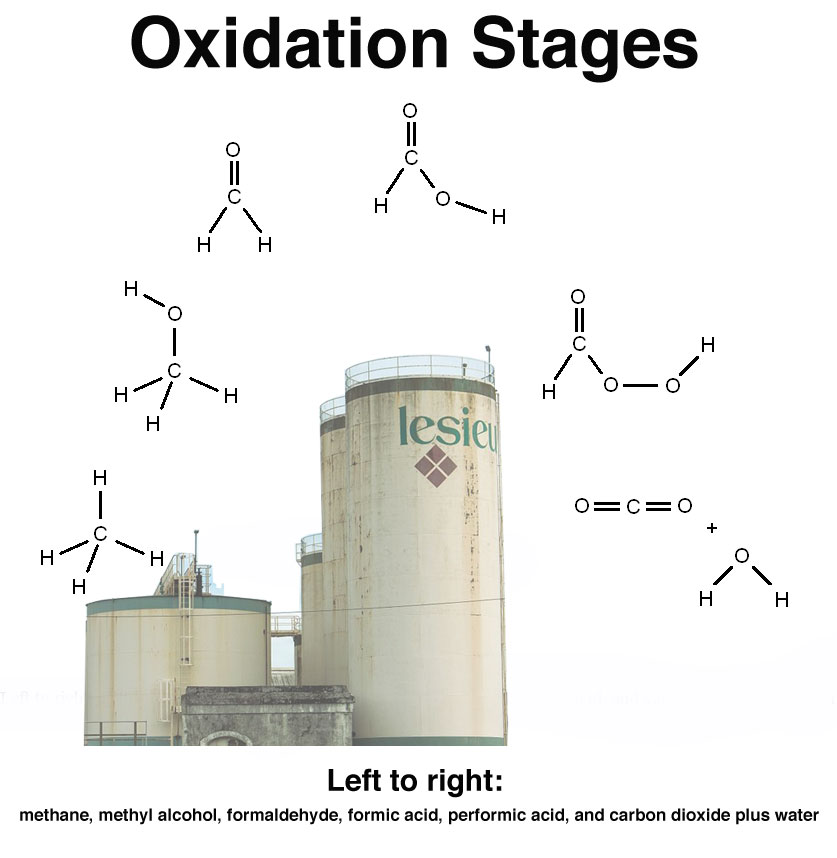
CH₄ → CH₃OH → HCHO → HC(O)OH → HC(O)OOH [→ CO₂ + H₂O ]
Their names (left to right) are methane, methyl alcohol, formaldehyde, formic acid, performic acid, and carbon dioxide plus water. A glimpse into their structures is seen in the associated image. Take special note of the single and double bonds between oxygen and carbon.
Oxidation of Methane to Methyl Alcohol
CH₄ + [O] → CH₃OH
The direct oxidation of methane to form methyl alcohol and stop there is very difficult to achieve. Methane resists chemical oxidation, whereas methyl alcohol readily oxidizes. Once the alcohol is formed, it is difficult to stop the oxidation of methane at that point. Yet, it is financially a very lucrative prospect for industry.
The process currently being pursued involves partial oxidation of the methane using heat and a catalyst. Although studies have been in progress for decades and the process yields some methanol, it is not yet suitable for production.
In turning attention to the laboratory, there is no immediate need for such a synthesis, as methyl alcohol of exceptional purity is fairly cheap and easily obtained.
Methyl Alcohol to Formaldehyde
CH₃OH + [O] → HCHO [+H₂ or +H₂O]
Unlike the conversion reaction for methane, above, the conversion of methanol to formaldehyde proceeds readily. But the same problem stands in the way. The product formaldehyde is easily oxidized to formic acid, HCOOH. So the oxidizing agent needs to be carefully controlled.
Industrially, two catalytic processes predominate. One uses finely divided silver. The other combines iron and molybdenum oxides. Both processes require heat. Another less lucrative approach is to use enzymes.
In the laboratory, if formaldehyde was not already inexpensive, two useful reagents would be acidified sodium dichromate (Na₂Cr₂O₇) or acidified potassium dichromate (K₂Cr₂O₇). These combinations produce the oxidizer chromic acid, H₂CrO₄.
CH₃OH + H₂CrO₄ → HCHO + CrO₂ + 2 H₂O
The catch is, the oxidant must be added gradually and never in excess, or the aldehyde produced will itself oxidize to produce formic acid.
Methanol or Formaldehyde to Formic Acid
Of course, by the above admission, if an excess of the dichromate oxidizer is added to the reaction mixer, the result will be a good yield of formic acid.
CH₃OH + 2 H₂CrO₄ → HCOOH + 2 CrO₂ + 3 H₂O
What about industrially, though? What is one of the preferred processes? Put simply, the selective oxidation over a supported vanadium oxide catalyst, with water and air at 120°C. The reaction may be written,
2 HCHO + O₂ → 2 HCOOH
Formic Acid to Performic Acid (and Beyond)
HCOOH + H₂O₂ → OCOOH + H₂O
This substance, a serious explosive, must be kept cold and not come in contact with oxidizable substances. It is prepared from highly concentrated formic acid and strong hydrogen peroxide (35% to 50%) solutions. Industrially, it is prepared in much the same way, but with the addition of a catalyst.
Performic acid is used in very specialized ways by the biochemist. It is highly valued in dilute solution for disinfecting, since its breakdown products consist primarily of harmless carbon dioxide, oxygen, and water.
Final Remarks
As was shown, the oxidation of methane can produce differing results, based on the strength of the oxidizer, its specificity, and way in which it is used. As a synthetic tool, just the one example of methane was discussed. In addition, no attempt has been made to discuss the broad spectrum of oxidizers. As an example, ozone can be used to cleave double bonds, leading to multiple products. Neither have we considered the use of the more mild oxidizing reagents such as ferric or cupric salts.
Note: You might also enjoy Acid-Base and REDOX (Oxidation-Reduction) Chemical Reactions
References:
- Stanford University: Direct Conversion of Methane to Methanol (2013)
- National Institutes of Health. NCBI: Biological Conversion of Methanol to Formaldehyde
- Springer Link. Catalysis Letters: The Selective Oxidation of Methanol to Formaldehyde…. (2002)
- Master Organic Chemistry. Reagent Friday: Chromic Acid, H₂CrO₄ (2016)
- Science Direct. Applied Catalysis: Selective oxidation of formaldehyde to formic acid over supported vanadia catalysts (2014)
- Springer Link. Performic Acid Oxidation (2016)

Converting methane to methyl alcohol, if it could be performed easily and cheaply would be a good way of removing this greenhouse gas from the atmosphere? Even allowing it to oxidize the full way to carbon dioxide would be an improvement, as I understand that carbon dioxide is not quite as potent a greenhouse gas as methane? But being able to stop it at methyl alcohol would be good, though I am not sure what use could be made of this?