
Is there a difference between soap and detergent? Both clean what they are made to clean. But, yes, there are differences. They come from different sources. They are different chemically. And they are put to different uses. An example of each is provided, below.
Soap
There are a variety of soaps. A soap is the metal salt of a fatty acid.
A fatty acid is an organic compound most often of animal or plant origin. A fatty acid contains a long-chain aliphatic carbon skeleton (with or without branches) plus a carboxylic acid group (-COOH) at its end.

The metal may be an alkali metal such sodium (Na) or potassium (K). These metals are found in the first column of the periodic table of the elements. Or, the metal can be an alkaline earth metal, such as calcium (Ca) or magnesium (Mg). These metals are found in the second column of the periodic table of the elements.

Detergent
Detergents have some similarities. But are often of synthetic origin. They are not made insoluble by hard (mineralized) water. Instead of a carboxylic acid group, detergents contains a more intensely ionic group. It may be a sulfate or a sulfonate group (-OS(O)2-OH).
In addition, detergents can include aromatic rings. Detergents can also be used as surfactants and foaming agents.
There are even detergents that dissolve in solvents other than water, such as gasoline. These often include nitrogen in their formulation. The nitrogen compound frequently includes a ring as part of its structure. Such compounds are not only detergents, but dispersants.
Example Difference Between Soap and Detergent
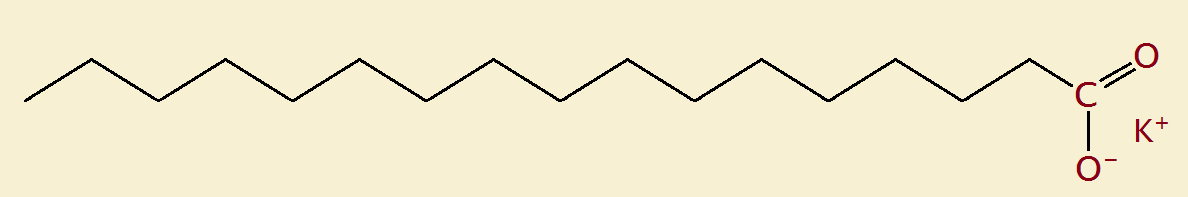
An example of a soap is potassium palmitate:
CH3(CH2)14-COO– K+
An example of a detergent is sodium lauryl sulfate: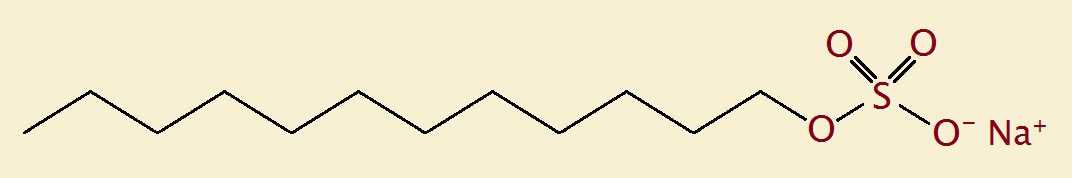
CH3(CH2)12-OS(O)2-O– Na+
Note: You might also enjoy Ammonia and Bleach – Why a Bad Choice?
References:

I’m a soap maker. Excellent article.
I did not know these facts. Quite fascinating.
In the UK, most shampoos and shower gels are detergents, as is what we term “washing up liquid”, which I think Americans call “dish soap”. My granddaughters love bubble bath (detergent) but it is so hard to get the bubbles to drain after the bath. I have found that washing your hands with a piece of soap in the detergent water helps get rid of the bubbles. They seem to work against each other!
Didn’t your daughters use soap in the bath to wash themselves?
Based on the article, they used detergent…
MegL is in the U.K. They may use slightly different methods to bathe. In the U.S., it is still often bar soap that is used for general hygiene.
In my experience, detergent is much better at dissolving fats, without leaving any residue. If you wash your hair with soap, you will find it very difficult to rinse thoroughly. If you have been in poison ivy and need to wash the irritant oil off your skin, detergent is far more effective than even soap made with lye. (East coast of the USA, I have years of experience on this point!). On the other hand, soap is much nicer! I use brown soap for wiping my kitchen sink and surfaces. But my question is still, in what way does detergent work differently from soap? What is the mechanism of the soap molecule vs. the detergent molecule?
Thank you.
I would also like to know the difference in the mechanisms of the two different types of molecules (soap vs detergent), so am asking one of you/someone to reply to that question. I just finished reading an article about garlic used as an insect repellant which specified using a soap rather than a detergent (for dogs), which is why I was looking for the difference between the two. So, while I liked the article, I don’t really have an answer to my original query about why a soap may be preferable to use in certain applications over a detergent. Also, – separate topic – What is the purpose of the italicizations in your article? The italicized words in your article seem to need definitions, but on my iPad there are no hyperlinks.
Many of the italicized words in the article are defined within the article. Those that are not can be understood by searching the word online. For instance, enter: Define: dispersant.
I don’t care for detergent but this article gave the right information on what the difference between the two is.
It was really helpful to know what is the difference between soap & detergent in a simple way.
Is it advisable to use a detergent just after production?
Please elucidate (explain further). Just after what is produced?
Molecularly, how do they differ in the cleaning process? My Dad once told me that one of them wraps the dirt (I think that was the soap) and lifts it up, and the other dissolves the dirt — which is which, and is this accurate in any way?
Thank you very much.
Patti
Soap ionizes in water. The fatty portion of soap attaches to the dirt. Micelles form around the dirt, enabling it to be all washed away. Detergents are stronger, synthetic chemicals that act primarily as surfactants. They loosen the dirt from the dirty object.
Is it advisable to use soap to wash laundry in a washing machine then?
It’s up to you, as long as you are careful to limit quantities so sudsing isn’t excessive. Personally, I’ll stick to detergent.
My small washer/dryer’s instructions say to clean the casing with soapy water or a non-solvent cleaning agent. What would a non-solvent cleaning agent be? Would they be referring to something like Murphy’s Oil Soap or a similar product without soap in the name? Do you know any common examples?
Murphy’s should be just fine. Although water is an excellent solvent, sometimes, as undoubtedly you know, language can be confusing! Generally solvent, in such a context, refers to something like turpentine, gasoline, an alcohol, a ketone, or other hydrocarbon substance. Some hydrocarbons can not only assist in cleaning, but in removing paint or dissolving plastic. You wouldn’t want to use those in the case you describe. It is true Murphy’s contains some glycol, but it primarily uses water. When in doubt, you can always clean a small spot that is not visible to test its efficacy.
Thank you so much for this!
Is it advisable to use detergent in place of soap for bathing?
Not necessarily. Soap works just fine.
Can I use soap in H[igh] E[fficiency] washers? [The] tag on [the] sleeping bag says [to] wash with soap, do not use detergent.
The issue with using regular detergents or soaps in a high efficiency washer appears to be degree of sudsing. You are ill-advised to use soap. However, should you choose to do so, you would need to be sure to use only a small quantity.
Great article. My grandparents always said to only use yellow soap because it’s the most natural/healthy one. Whether it’s true… I don’t know. But as someone with very dry skin especially on hands, majority of soaps, shampoos and other hygiene products are a death sentence to my hands. Even hot and warm water are drying them. That’s why I only do quick cold showers, and use soaps only during them. I wash plates with special one-time use gloves (though i use them for months) and it makes my hands less dried. I always wonder if I should use soap on my face or not during shower, because I usually clean it with water only to avoid getting a little bit in my eye, which is iching a lot and causing eye redness.
I’d heard the same thing about the Fels-Naphtha type of soap. As to bathing with soap, our Dermatologist was reputed to have said he washed his skin with soap only in the folds. Doubtless to kill any breeding bacteria. The closeness, dark, and damp within fold areas would make an excellent breeding ground for bacteria.
In using soap to make fire ant mound killing drench (without poisoning the earth), usually “Dawn” brand dish-washing liquid is specified.
What I really want to know is can I substitute a cheaper dish-washing liquid? What about “garage floor cleaner”? Both claim to “cut through grease”.
My experience in keeping house for nearly forty years has been that soap takes food grease out of cotton material better than detergent does. If I find an oil stain or a patch soaked with melted butter, I could wash it in detergent a dozen times and it would not come out entirely. The way I get it out is to wet the spot with very hot water and rub bar soap into it (plain Ivory works very well) until the stain is saturated with soap. Then when it’s later laundered it comes out cleanly with no trace of the grease. This contradicts what is said here about detergents, but it’s what I have found to be the case for many, many years.
Situations, detergents, water, etc. vary. You say you rub soap into the fabric. I doubt you do that with the detergent?
I say you are fine with skipping the soap, especially for your face. Some people think this is actually better for you, that excessive use of soaps takes the natural oils out of your skin, which advances drying out and wrinkling, and general aging. This is especially important on the face, obviously. I know many people will cry horrors, bu the fact is that water is an excellent solvent and cleanser, and whether you are doing dishes, washing clothes or bathing, 95% of the cleaning comes from the water alone. The only real difference is in getting rid of grease, which mostly applies to washing hair. And again, some people insist that some greasiness is natural and healthy, and this idea of having perfectly clean hair and skin (and bathing every single day, for that matter) is an idea that has only existed for the last 100 years or so. I’m convinced there is some truth in the claim that daily bathing is excessive and unhealthy; every three days, or even weekly used to be the standard, and still is in many areas of Europe.
When I wash dishes, I find that excepting really heavy grease, they wash perfectly fine with nothing but good hot water. It may leave a very thin film of grease on the dishes, but that doesn’t bother me. I’ll wash them with soap periodically for the sake of it, but the claims that it’s “unhygenic” don’t bother me. We live surrounded by bacteria, no matter how clean you try to make things, and I have never, ever heard of someone getting sick from eating off a plate that wasn’t perfectly cleaned because “bacteria” grew on it.
Even laundry detergent makers say that people tend to use far too much. Most people just habitually use a full cap of detergent for a load, but in fact they say that straight water will wash the majority of clothes, and 1/4 or 1/3rd of a cap should be more than enough to clean all but the greasiest or most stained clothes. Using a full cap of modern concentrated detergent is usually too much, and it just leads to clothes that don’t rinse properly, or a waste of detergent at best.
Anyway, point is that you needn’t worry much about skipping the soap on your face. In theory this will make your pores all big and clogged with oil (says the marketing people), but my experience has been the exact opposite. I think washing with soap too frequently DOES clean out the pores….which then fill back up even worse than before. They “stretch out”, or seem to. Whereas a good washing with water, maybe a little mild soap now and then seems to allow the natural oils to do their thing, and the pores just stay the way they are supposed to stay. Maybe I’m totally mistaken, but I’m in my mid 30s and people usually mistake me for 20 or so. But maybe that’s just my naturally youthful good looks? O.o No one avoids it forever.
And of course when you wash your hair, there will likely be a certain amount of soap and water that runs down across your face anyway, so that should help, even without actually washing your face directly. There isn’t really any serious difference between body wash and shampoo, other than packaging and maybe additives. One can use either one for al all purpose cleaner and it works just fine, if you are just going for generally clean.
Under normal conditions, at least to some extent, I agree with what you say. Of course, at the moment (with Covid-19), it is of value to “take no chances” when it comes to cleanness and cleanliness.