Solids may be subdivided into amorphous solids and crystalline solids. Amorphous solids possess limited order in the way molecules are bonded to each other. Crystalline solids, on the other hand, exhibit an exceptional degree of order.
Logic should tell us a mixture of crystalline solids should be capable of chemical separation and purification through some reiterative crystallization process, based on relative solubilities.
This proves to be true. The process is called fractional crystallization.
Before discussing fractional crystallization, it might prove wise to discuss the simpler process of fractional distillation, the separating by distilling of a mixture of liquids possessing markedly different boiling points.
Ordinary Distillation
Consider an example of two liquids, Component A and Component B, that are miscible (they dissolve completely one within the other). The immediate goal is to separate A and B. But their boiling points are not all that different. A boils at 138° C, whereas B boils at 149° C. Although A boils first, some of the B molecules are energetic enough they also boil over. The end result is two fractions, one rich in A, the other rich in B. But the A fraction contains some B and the B fraction contains some A.
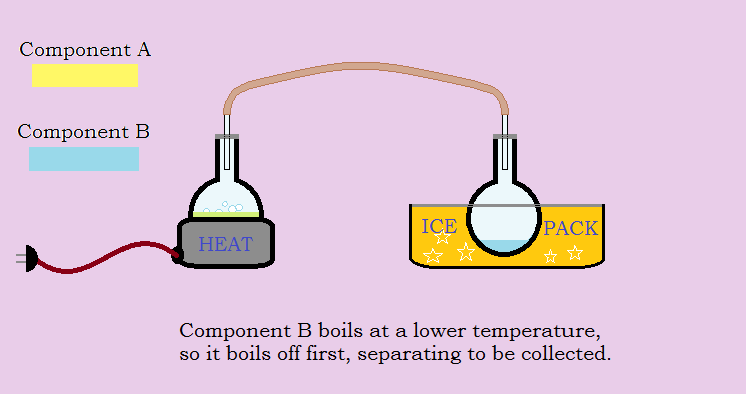
Distilling the two resulting fractions to produce four fractions and combining the two B-rich portions with each other and the two A-rich portions with each other improves matters, but it does not achieve total separation. It would take many such distillations to do the job.
Because of all this, simple distillation is usually limited to component mixtures that exhibit a minimum of 25° C boiling temperature difference.
A smaller differential calls for the alternative, fractional distillation.
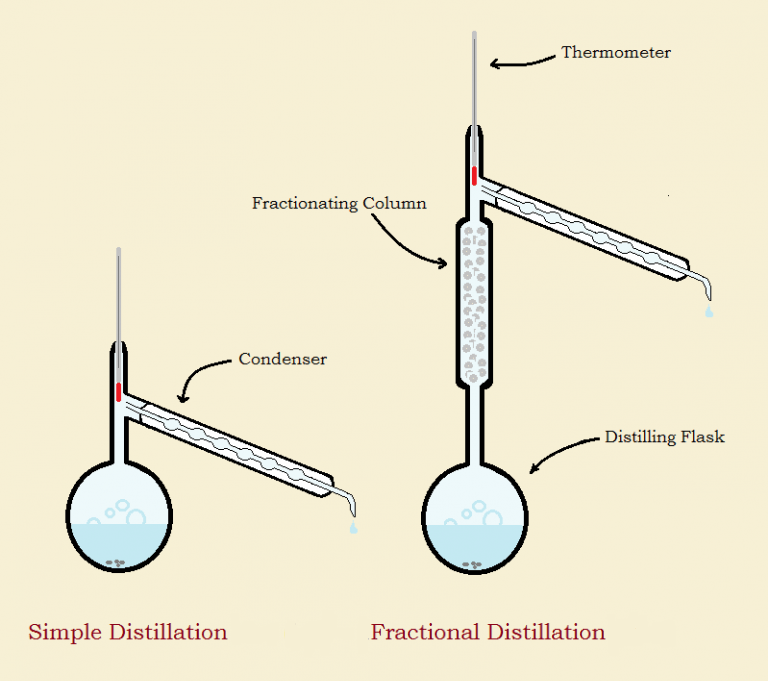
Fractional Distillation
Fractional distillation is essentially equivalent to multiple simple distillations. In fact, if one wishes to specify the quality of a fractional distillation, it could theoretically be characterized in terms of the number of simple distillations it is comparable to. As the image shows, fractional distillation utilizes two condensers (glass attachments that turn vapor to liquid).
The first condenser is sometimes called a “packed column,” though occasionally “fractionating column” or some other term is used. Such columns are filled with blockages such as glass beads, intruding glass nipples, or spiral tubing. Sometimes, a spinning band of metal or Teflon has been used that pushes escaping vapors back toward the distillation flask.1 Occasionally the outer glass jacket is encased in insulation or in insulation with electrical wires for carefully controlled, minimal heat application. What is the purpose of these?
The vapors cool and condense as they rise, but the vapors are not completely of just one component. The higher boiling component tends to condense first. The blockages provide surfaces for the vapors of that component to liquefy so it will return to the flask as condensate while the lower boiling vapors succeed in escaping.
Any applied heat is to maintain this action, especially in the lower and middle portions of the packing. The better the equilibrium achieved, the more the higher boiling fraction is kept from distilling over. The end result is chemical separation and purification by fractional distillation. The trick is to maximize the efficiency of the packing to provide a complete separation.
Crystallization
The routine crystallization of solids from solution behaves in comparable fashion to ordinary distillation. The solid has a particular solubility in the solvent it is dissolved in. Evaporate the solvent, and the solubility of the solid is overpowered. Some solid must come out of the solution, and it does so by forming crystals. The more solvent that evaporates, the more solid that forms crystals. No problem.
No problem, that is, unless there is more than one crystalline solid in the solution. How can they be separated? The more-soluble compound of the two begins to crystallize out first. If there are two components that have considerably different solubilities, simple crystallization may prove useful. Yet, as was the case for distillation, the problem becomes difficult if component solids have similar solubilities.
One extreme example of this involves the rare earth metals. These elements are so similar, one to the other, that the solubility of their equivalent compounds makes separation by simple crystallization impossible.
There is another extreme example that is of historic significance. It was the isolation of radium from the ore pitchblende, as performed by Marie and Pierre Curie.
Fractional Crystallization
Many tons of pitchblende must be processed to obtain even one gram of radium. The extraction yields two major fractions. Polonium resides in the bismuth fraction; radium resides in the barium fraction. The ratio of radium to barium proved to be a mere 3 parts in 100,000. How could chemical separation be achieved?
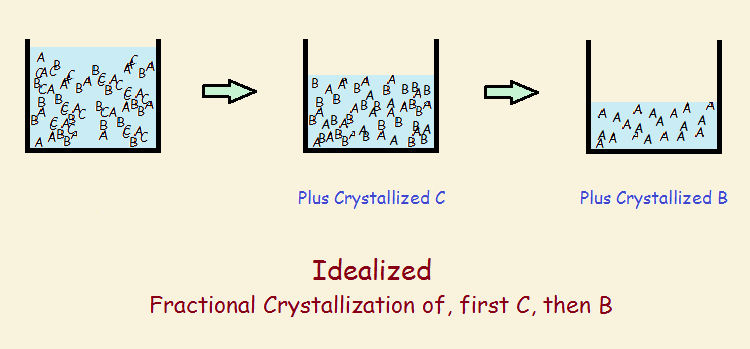
The method chosen was isolation by fractional crystallization. But first, the insoluble sulfates had to be converted to soluble form. The chlorides were chosen, radium chloride being less soluble than barium chloride.
Fractional crystallization involves dissolving the salt mixture in water, concentrating the solution by evaporating the water, and allowing some crystals to form. The first crystals would incorporate the most radium. Many collections of these crystals would be collected together and dissolved.
The first crystals forming in this crystallization would be even higher in radium. However, to quote Madame Curie,
“To obtain a very pure salt I… had to perform several thousands of crystallizations.”
The multiple application of a process to achieve a suitable result is termed a cascade process. In this instance, chemical separation and purification were achieved by fractional crystallization.
Strategies for Chemical Separation and Purification
Although there may be subtle strategies to enhance the procedure of fractional crystallization, clearly it is not so efficacious a procedure as is the fractional distillation of liquids. Yet, ultimately, where the tiniest of differences exist in chemical or physical properties, such tools as these must remain in the chemist’s toolbox.
1 See Spinning Band Distillation
Note: You might also enjoy Imidazole Synthesis and Chemistry
References:
- Wellesley College. Fractional Distillation
- Nobelprize.org. Marie Curie – Nobel Lecture: Radium and the New Concepts in Chemistry (1911)
← Back to Classic Science
← Home

I first heard of fractional distillation in school. Maybe they don’t do that these days. But I never heard of fractional crystallisation.
Yes, indeed. You really ought to watch the phenomenal movie about Madame Curie, starring Greer Garson and Walter Pidgeon. Fantastic! You can find it online…