 Graphene. What is it? Yet another variety of this stuff…
Graphene. What is it? Yet another variety of this stuff…
Black chunks of consist largely of carbon. However, there is a wide variety of carbon varieties. How many are surprising.
The list of known varieties is growing. These include soot, diamonds, graphite,¹ and the curious buckminsterfullerene or bucky balls. In addition, there are tiny carbon nanotubes, a hot item to researchers. Despite these amazing forms, perhaps the most fascinating carbon discovery was to come. That form is the graphene sheet.
Discovery
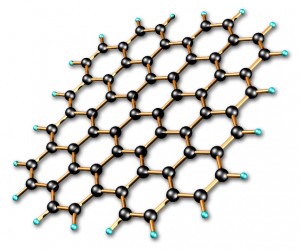
Graphene is a super material. For its successful isolation, André Geim and Konstantin Novoselov, were awarded the 2010 Nobel prize. Each sheet is one atom thick. It resembles a honeycomb. Yes, it looks like chicken wire.
First Production
The first effort at isolation involved thinning a pad of graphite. Adhesive tape was attached to the sides of the pad it was pulled apart. In time, a single layer was left. There are two easy ways to visualize this.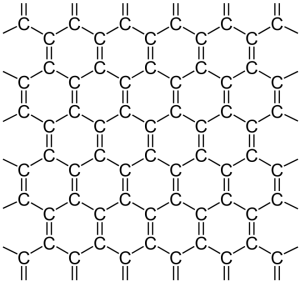
First, imagine a flaky biscuit with many layers. You peel the layers off, then eat them. Second, a child has a piece of flaky mica. This can be peeled in the same way as the biscuit.
Now since the layer of carbon is only one atom thick, special steps are need to study it! In order to examine the layer, it must be stabilized. To help do this, it is attached to a substrate.
Silicon dioxide was chosen. Once a layer was attached to it, the tape was dissolved off the carbon using a solvent. This led to the adjoining of the layer with the silica. The sheet did not curl but remained flat!
Graphene Properties and Applications
In x-y or 2-d space, the bonding between carbon atoms is sigma (σ). Electrons direct their charges along the bonds within the carbon sheet, itself. The remaining available electron—one for each atom—lies in the z- or third dimension, above and below the sheet. Those electrons are pi (π) electrons. They are highly mobile.
Mobile electrons in motion are electricity. Honeycombed carbon electron orbitals overlap. They form a “conduction band.” This effect is heightened by special principles of physics. For further discussion, see the MIT reference below.
By creating holes within a graphene shee and “doping” the holes with chosen impurities, semiconductors can be made. These are nearly unbreakable. They are highly flexible.
Could There be More?
Researchers at the University of Texas point to some future applications. Among these are transparent electric conduction. Imagine heating your windshield with a fabric made of carbon. Structural devices could be made. Examples might include turbine blades, electrical energy storage, catalyst support, and composites. Others suggest the honeycomb carbon would be ideal for terahertz electronics devices.
Improving Production
This way of isolating a graphene sheet needs adjustment. Thermal exfoliation may be an alternative, says Princeton University. ² Some have suggested chemical methods.³
¹ In fact, graphite consists of layers of carbon that are not attached, but slip across each other—each layer being a graphene sheet.
² Princeton University Ceramic Materials Laboratory: Commercial Production of Graphene
³ See, for instance, A Chemical Route to Graphene for Device Applications and Large Scale Graphene Production Using a Radio-Frequency Catalytic Chemical Vapor Deposition Method.
References:
- Lawrence Berkeley National Laboratory: Image
- National Science Foundation: Graphene (video)
- BBC News: Is graphene a miracle material?
- MIT: Graphene – Electron Properties and Transport Phenomena
- University of Texas: Graphene – the Road to Applications
Note: You might also enjoy What is an Aerogel?

[…] Info Source: Reuters | Image Source: Wikipedia and QuirkyScience […]
[…] Graphene Properties and Applications – Isolation and Production […]