 What is a hydronium ion? Water… Is there a more important or abundant liquid on the face of the earth? It possesses an amazing number of properties. It is essential to our very existence.
What is a hydronium ion? Water… Is there a more important or abundant liquid on the face of the earth? It possesses an amazing number of properties. It is essential to our very existence.
Water is one of an extremely small number of substances (liquid ammonia is another) that expands and becomes lighter on freezing.
Ice floats and forms an insulating, protective crust over underlying water. If ice sank rather and didn’t float instead, the ponds and lakes of earth would completely freeze. All living things would die.
Structure & Electrical Properties of Water
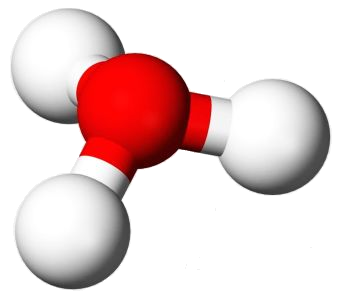
Water’s chemical formula is H2O. Occasionally it is written HOH or H–O–H. This way of writing the structure is convenient as long as one is aware of the shortcomings. Water is not a linear molecule. It is shaped like the head of a mouse. The much smaller hydrogen atoms are the ears. Oxygen is the head.
Water is polar because it is non-linear and is constructed of elements of very different electronegativity. That means that there are portions of the water molecule that possess partial charges, even though combined they add up to zero.
Interaction with Ionizing Acids
One of water’s most interesting properties is its interaction with ionizing acids. Consider the example of hydrobromic acid. Hydrogen bromide gas is not ionic. When dissolved in water, however, it becomes hydrobromic acid. This is because it dissociates into ions when combined with water. We may write this dissociation,
HBr → H+ + Br⁻
but this is not really accurate. More correctly, we write
HBr + H2O → H3O+ + Br⁻
H3O+ is called a hydronium ion. It is “protonated” water, since a positive hydrogen ion is actually a proton.
Notice that the same positive charge is now held in some fashion by three hydrogen atoms instead of one. This means each separate hydrogen atom holds only 1/3 of a charge on average. Charge delocalization – spreading out the charge – increases the stability of a system.*
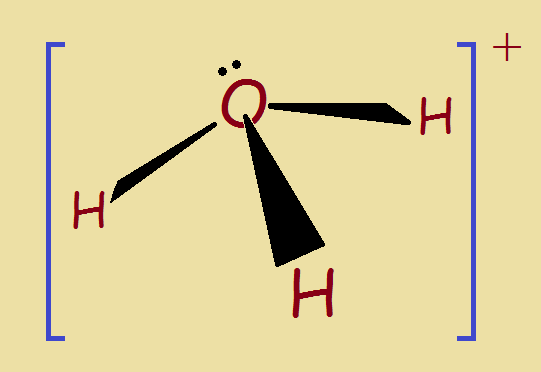
Shape of the Hydronium Ion
In many ways, the hydronium ion resembles an ammonia gas molecule, NH3. Ammonia is a trigonal pyramid. Its hydrogen atoms are like the legs of a tripod. The nitrogen is like its top. The camera is like an unbound pair of electrons above the nitrogen.
A similar situation exists for the hydronium ion. It too has an unbound electron pair above the oxygen. For this reason, hydronium is not flat. Each H–O–H bond angle (113o) differs from the H–N–H bond of ammonia (107.8o). The hydronium tripod is more open than the ammonia tripod.
Its Importance
As we have seen, the water molecule allows acids to ionize. This is possible because of the formation of the hydronium ion. This is of immense importance not only to the physical properties of the universe, but to life itself.
 Beyond the Hydronium Ion
Beyond the Hydronium Ion
Still and all, this picture is not 100% complete. While the hydronium ion contains the hydrogen ion in its structure, the hydronium ion itself is surrounded by yet more water molecules.
This serves to spread the positive charge further, stabilizing the system to a greater extent. The number of molecules associated with a given hydronium ion can range from perhaps six to many more than a dozen.
* For further information on this, see the author’s article, Ions are Stabilized by Spreading the Electric Charge
Note: You might also enjoy Hydrogen Bonding Effects at Various Levels
References:
← Back to Classic Science
← Home

What are the physical characteristics of hydronium – liquid at (temp range); stability at (temp range); Freezing and boiling point, etc.?
[…] What is a Hydronium Ion? […]
[…] water molecule allows acids to ionize. This is possible because of the formation of the hydronium ion. This is of immense importance not only to the physical properties of the universe, but to life […]
[…] water molecule allows acids to ionize. This is possible because of the formation of the hydronium ion. This is of immense importance not only to the physical properties of the universe, but to life […]