 Ever purchased wipes or some other product that was labeled hypoallergenic? Why did the label say that? Hypoallergenic is defined as: relatively unlikely to cause an allergic reaction. So all the ingredients in such a product must have a well-established safety record. Even if a few individuals experience difficulty, it would be mild, perhaps superficial.
Ever purchased wipes or some other product that was labeled hypoallergenic? Why did the label say that? Hypoallergenic is defined as: relatively unlikely to cause an allergic reaction. So all the ingredients in such a product must have a well-established safety record. Even if a few individuals experience difficulty, it would be mild, perhaps superficial.
There is a compound that, despite being hypoallergenic, fights mold successfully. Its name? Iodopropynyl butylcarbamate.
The achieved objective requires only a very slight amount of IPBC. Effective at minimal concentrations and water-soluble, IPBC is a cost-effective anti-fungal preservative.
A Closer Look at IPBC
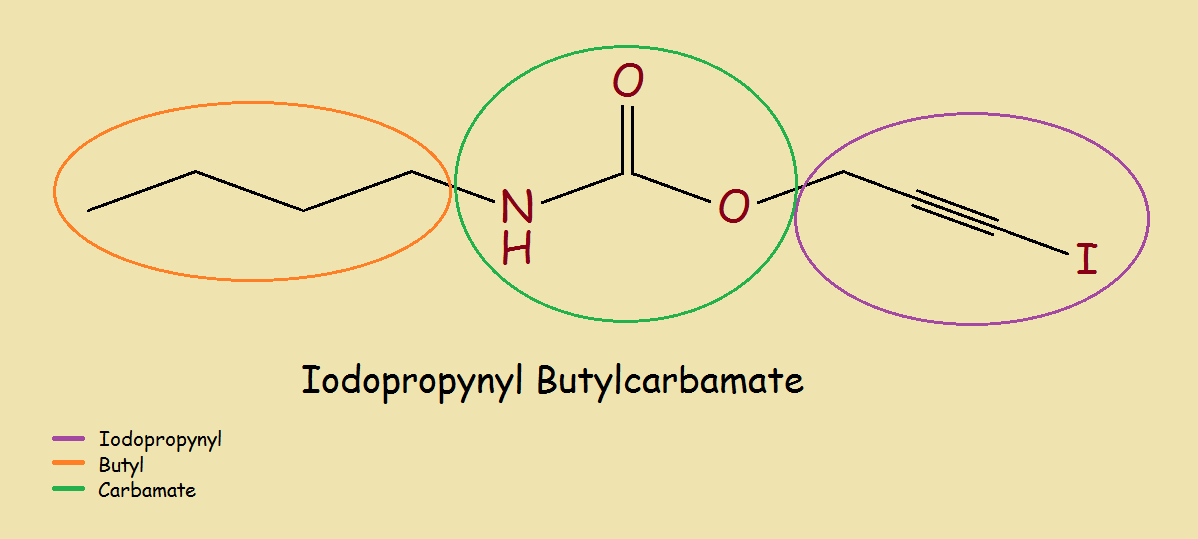
Notice the chemical structure of iodopropynyl butylcarbamate in the illustration. The portion of the molecule encircled by green is the carbamate portion of the molecule. It is a derivative of carbamic acid (H2N-COOH). So IPBC is “related” to the carbamate biocides.
The portion of the molecule within the purple circle is the iodopropynyl part. The triple bond is found in its simplest form in the acetylene molecule (H-C≡C-H). Propyne is H3C-C≡CH. Iodopropyne is H3C-C≡C-I.
The part in the orange circle is the butyl part – very similar to butane (CH3CH2CH2CH3). This part of the molecule increases the molecular weight and size by 3 carbon atoms and 9 hydrogen atoms. High molecular weight assures low volatility.
Low Volatility IS Important
The low volatility of iodopropynyl butylcarbamate is important because biocides can be toxic if inhaled. In powder form, IPBC would be quite toxic if inhaled.
Note: You might also enjoy BHA and BHT in Your Food – What are They?
References:

That’s interesting, I didn’t realise that hypoallergenic meant the killer stuff was still there but in very mild concentrations!