
Citrus fruits offer both pronounced flavor and pungent aroma. Where does the pungency come from? From the terpene limonene – more specifically, the dextrorotatory enantiomer¹, d-limonene.
Although modern terminology is switching from (D)extro and L(evo) notation to S(inister) and R(ectus), we’ve chosen the older terminology, more often associated with limonene.
What is Limonene?
Limonene is useful commercially in environmentally-safe cleaning formulations, as a solvent, and as raw material in the synthesis of other chemicals, such as carvone.
This chemical is also useful as an insecticide and for assorted other purposes. Manufacturers separate limonene from the fruit’s peel by means of steam distillation, or a centrifugal process.
Lab Synthesis of Limonene Citrus Cleaner from Isoprene
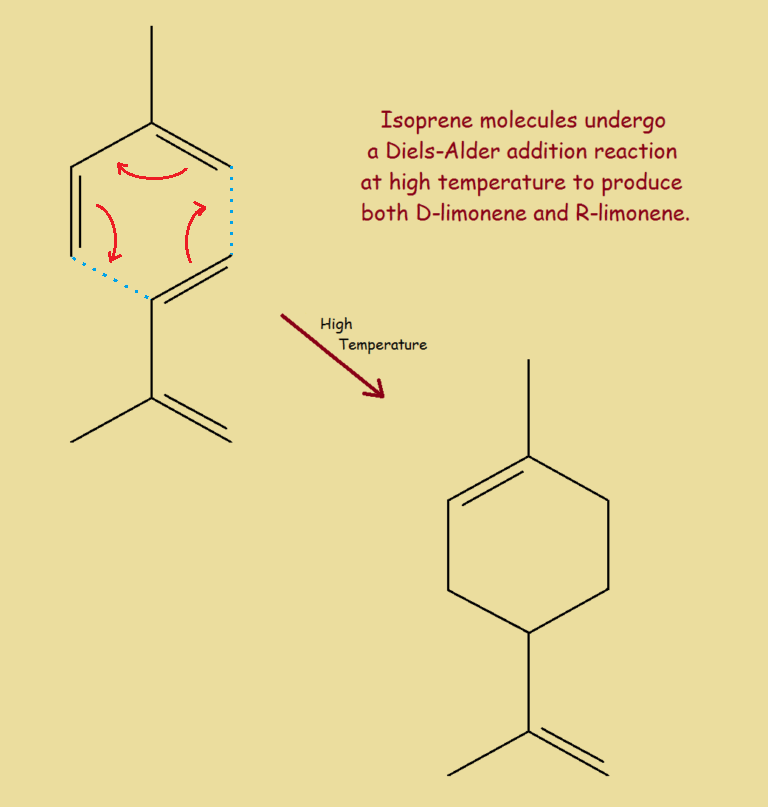
Labs can prepare limonene (a mixture of both enantiomers) from two molecules of isoprene (a starting material associated with the manufacture of tires).
Since this process involves the joining together of two molecules, with nothing eliminated, it is an addition reaction – classified as a Diels-Alder reaction.
Limonene in Nature
In nature, terpenes are not derived from isoprene. Rather, they come from isopentenyl pyrophosphate.
Isopentenyl pyrophosphate has the structural formula:
H₂C=C(CH₃)CH₂CH₂-OP(O)O-O-P(O)OH
Isopentenyl pyrophosphate is derived beforehand by a complex series of metabolic steps identified as the mevalonate pathway.
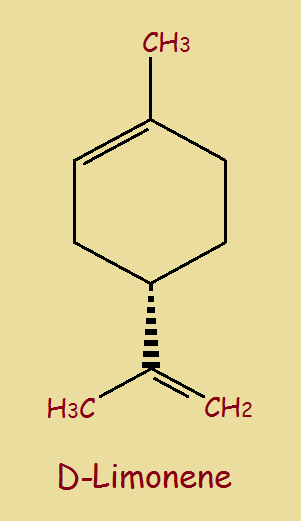
This is a stick model of D-Limonene, a simplified representation. The vertices represent carbon atoms. Hydrogen atoms are implied, not drawn. The dashed line means the part at the bottom lies beneath the plane of the paper, while the remainder of the molecule lies within the plane.
D-Limonene to L-Carvone
Levorotatory carvone (l-carvone) is a substance that exists in many essential oils, but a lab can use dextrorotatory limonene (d-limonene) to prepare l-carvone as well – typically for flavoring or fragrance.
First, they react d-limonene with nitrosyl chloride to form d-limonene nitrosochloride.
The nitrosochloride then reacts with potassium hydroxide to form l-carvoxime.
The oxime is further reacted with dilute acid, giving l-carvone.
L-carvone is a terpenoid (terpene derivative) with the aroma of spearmint. Interestingly, however, d-carvone does not smell like a variety of mint – but like caraway.
Limonene as Raw Material
When processing citrus fruits to harvest limonene, according to the Florida Chemical Company, Inc. website, nothing from the fruit used (generally oranges) goes to waste – not the pulp beneath the peel, not the seeds. Processors use vacuum distillation to produce food-grade limonene, and steam distillation to produce technical-grade limonene – for use in cleaners and so on.
Chemistry of Limonene
Limonene is a terpene (the word ‘terpene’ comes from ‘turpentine’) – but this pungent chemical doesn’t smell like turpentine.
It’s the source of citrus flavor and fragrance – whether in desserts as a food-grade chemical, or in cleansers – or to produce other flavors and fragrances via the use of chemical reactions.
Love the smell of citrus in cleansers and flavorings? Now you know where it comes from.
Note: You might also enjoy Oxalic Acid in Your Food
References:
- Reed College. Laboratory Manual – Synthesis of Limonene
- Columbia University. The Isoprene Rule
- New Mexico Institute of Mining. Steam Distillation of an Essential Oil
- Scripps. Classic Terpenes Syntheses
- ACS Publications. L-Carvone from D-Limonene
- Diels-Alder I. Mechanism
- Diels-Alder II. Endo vs Exo
- Diels-Alder III. Stereochemistry of Dienophile
- Diels-Alder IV. Stereochemistry of Diene
- Florida Chemical Company, Inc.. Inside Florida Chemical
- Science Madness. Limonene
