 In chemistry, many reactions are simple, intuitive, and straight forward. Others can fool the uninformed. Organic pericyclic reactions are included among the latter.
In chemistry, many reactions are simple, intuitive, and straight forward. Others can fool the uninformed. Organic pericyclic reactions are included among the latter.
To illustrate this, we consider two reactions. The first is a simple esterification reaction, leading to the obvious product. The second is a pericyclic reaction.
The Esterification
Reacting an alcohol with a carboxylic acid generally produces a simple ester. So in the presence of a dehydrating agent, one can react ethyl alcohol with acetic acid, to get the ester ethyl acetate plus water.
CH₃COOH + HO-C₂H₅ → CH₃COO-C₂H₅ + H₂O
Organic Pericyclic Reactions
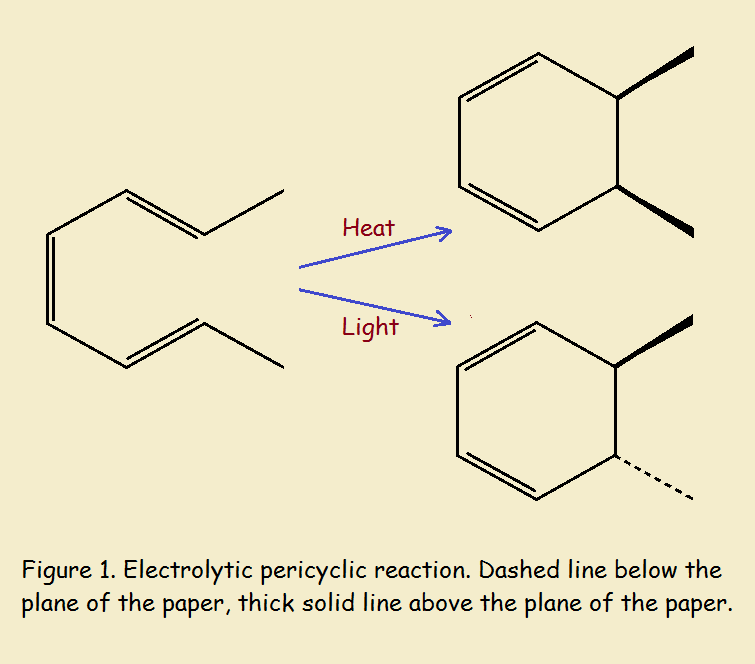
CH₃-CH=CH-CH=CH-CH=CH-CH₃ → two possible ring structures
The illustration in Fig. 1 shows a straight chain organic hydrocarbon converting into one of two cyclic structures, depending upon the source of energy.
The input energy, whether heat or light, enables the bonds between carbon atoms to rearrange. There is no change in their order. Pericyclic reactions of this sort, electrocyclic reactions, always involve a ring transition, or, in-between, state. The reaction is concerted.¹
In this particular case, the cyclic structure remains as the stable product. Notice that there is a total of ten bonds between carbon atoms in both reactant and product structures. The new bond formed in ring closure is a single sigma bond. The one broken is a pi-bond (one half of a double bond).
Other Pericyclic Reactions
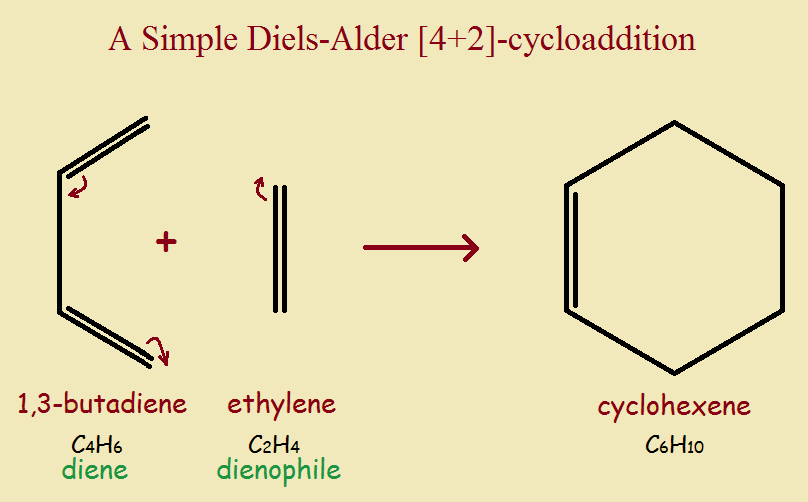
Most pericyclic reactions are thus rearrangement reactions. They involve a change in the bonding of framework. However, there are also pericyclic addition reactions. Such pericyclic reactions may be exemplified by the following:
CH₂=CH-CH=CH₂ + CH₂=CH₂ → cyclohexene [Fig. 2.]
This involves the formation of a ring, plus another single bond formed, giving a total of two new sigma bonds formed. Two pi-bonds are broken.
Finally, there is the simple sigma rearrangement pericyclic reaction, in which there is no net change in the number of sigma bonds or in the number of pi-bonds. See Fig. 3
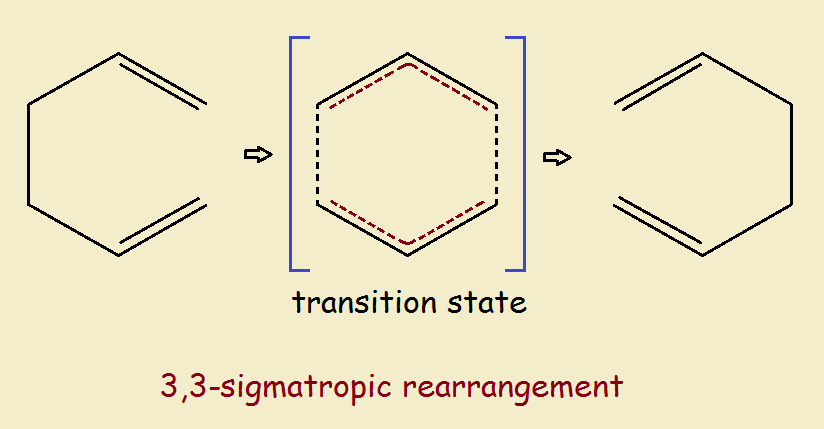
1 a concerted reaction is one in which all bonds are broken and made in a single step.
Note: You might also enjoy Epoxide Ring Preparation By Oxidation of Alkenes
References:
- Penn State University: Pericyclic Reactions
- Imperial College: Practice Problems in Pericyclic Chemical Reactions
- Michigan State University: Pericyclic Reactions
- University of Liverpool: Introduction to Pericyclic Reactions
← Back to Classic Science
← Home

Hey Bro, I am passionately fond of [the] writings on your website. They are made properly, easy to digest and understand, despite English being my 3rd language. All the best.
Thank you kindly, Charles. I try. I really do.