
Cutting-edge science is fascinating. Yet the science of everyday life is anything but boring. Consider the simple act of boiling water on the kitchen stove. There are many factors that come into play leading to the production of steam. Let’s take a close look at water as an individual molecule and as a cluster of interacting molecules.
Water at the Molecular Level
Water consists of one oxygen atom plus two hydrogen atoms. An atom of oxygen is much larger than one of hydrogen. Most hydrogen atoms consist of a lone electron in orbit about a single proton nucleus. Oxygen atoms have a much larger nucleus orbited by 8 electrons. Oxygen has a strong affinity for electrons. So it is an electronegative element. On the other hand, hydrogen readily donates its one electron. It is electropositive.
O + 2 e⁻→ O-2
H → H+ + e–
Oxygen (O), ordinarily assumes a diatomic (two atom) molecular form — (O₂). Hydrogen likewise assumes a diatomic molecular form. So we write our equations for the formation of water from the elements,
O2 + 4 e– → 2 O-2
2 H2 → 4 H+ + 4 e⁻
Combining these two gives us,
2 H2 + O2 → 2 H2O
It might seem reasonable to assume the hydrogen atoms in a molecule of water exactly oppose each other as H‒O‒H. In reality they do not. The water molecule is bent at approximately a 104.5° angle. This detail introduces an unequal distribution of charge we call polarity.
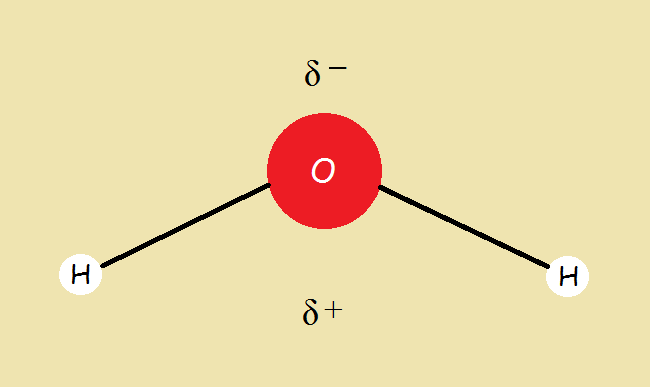
Polarity
View the bent water molecule by placing the oxygen at the apex and the two hydrogen atoms at the bottom. The oxygen has a slightly negative charge on its upper side, while the hydrogen atoms each have a positive charge half as strong as that of the one oxygen atom on their lower sides. It can be symbolized as residing between the two hydrogen atoms and below the oxygen atom.
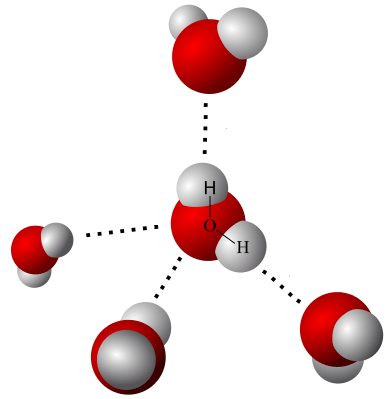
What Is a Hydrogen Bond?
The reader is surely familiar with the North and South poles of a permanent magnet. Electrical charges act in similar fashion. The polarity of water molecules draws other water molecules toward itself. These attachments are called hydrogen bonds.
The strength of such a bond is not nearly as strong as the force in a full chemical bond. To represent that fact, hydrogen bonds are drawn as dashed rather than solid lines. Since the pull is weaker, hydrogen bonds are longer, as well.
How Important Are Hydrogen Bonds?
Though weaker than full chemical bonds, it requires energy to disrupt hydrogen bonds. Collectively, this energy is considerable. This is why the boiling point of water, 100° Celsius, is as high as it is. The very similar molecule hydrogen sulfide (H₂S) has much weaker bonds between the hydrogen atoms of one molecule and the sulfur atoms of other molecules. Surprisingly, hydrogen sulfide at ordinary temperatures is not a liquid at all, but a gas that boils at -60° Celsius!
Additional Factors
There are other factors to consider, inherent to the water from your spigot. There are traces of soluble minerals and organic substances. Too, there is dissolved air. And don’t discount tiny dust or even rust particles. These all affect the temperature at which water boils.
The saucepan, whether glass or metal, possesses tiny sharp-edged imperfections – scratches or dings. These irregularities provide sites for bubble formation and release. This helps prevent super heating. Air and water vapor bubbles quickly form. They do not grow large before they are released from bottom and side surfaces.
Boiling Water
Place some water in a saucepan and place that on the stove – say a gas stove. Turn on the gas. What happens? The first thing that takes place is that the bottom of the saucepan heats up. As the water warms, a little turbulence results. This is because density decreases with rising temperature and the hottest water (that nearest the burner) is lighter than the water above it. Hence it rises to the top. The same principle causes a hot air balloon to rise in the atmosphere.
A Balance of Forces
Life would be simple if everything went one way. To heat water in a saucepan is to provide it with energy that increases the motion and disrupts the hydrogen bonds so the molecules can transform into the gaseous state. But there is another force in opposition to all this. It is the force of the atmosphere weighing down on the saucepan of water, which force is directly associated with gravity. This force opposes that introduced by heating.
Earth’s atmosphere rises from the surface of the ground hundreds of miles, though the bulk of it lies within the first ten miles. If one saucepan of water at the surface of our planet is heated in order to boil the water, another similar saucepan of water a few miles up will boil at a considerably lower temperature, since the force imposed by earth’s atmosphere is considerably less there. Try 72° Celsius at the higher elevation compared to 100° Celsius at the lower.
Boiling Water: Where Do Bubbles Come From?
But back to boiling water. Once we turn on the burner, it doesn’t take long before bubbles form at the sides of the pan. The first bubbles are air formerly dissolved in the water. With increased heat, molecules of water vapor form. As these coalesce through turbulence, they rise, and escape into the atmosphere. The escaping molecules are the most energetic ones, so the average energy of the remaining liquid statistically decreases. Something like this happens to animals and humans. When they sweat and it evaporates, the individual is cooled, to his or her relief. The Khan Academy video below explains how evaporation results in cooling.
When Water Boils
Thus, loosely put, water, as it is heated, becomes turbulent, and gives up its dissolved air in the form of bubbles. Then intermolecular hydrogen bonds begin to break apart and molecules of water change from liquid to vapor form. The vapor coalesces into water vapor bubbles, which in the turbulence coalesce to form larger bubbles. These are prevented from escaping into the atmosphere until the force of the atmosphere, caused by gravity, is overcome, the bubbles escaping. This adds to the turbulence of the water. We call this ever increasing turbulence bringing water to a rolling boil.
Note: You might also enjoy Tongue Stuck to a Silver Spoon Eating Ice Cream?
References:
- UCSB Science Line: What causes bubbles to form when boiling water? Where are the bubbles coming from?
- Purdue University: Boiling
← Home

Excellent explanation in minute details. Wish you most and more.
Thank you so much for your commendation.
Thank you for your excellent explanation! I can only suggest perhaps more explanation of the equation symbols for folks without a knowledge of chemistry syntax e.g. why oxygen has eight electrons but is designated as O⁻²?