
Oxygen atoms are very reactive chemically. We call them electrophilic or “electron loving” because the oxygen atom seeks to bind to other electron-rich sources, especially itself. Thus oxygen is present in our atmosphere primarily as the molecular O₂.
Another Form
A small percentage of atmospheric oxygen exists in another form, ozone, O₃. This less stable form of oxygen is produced by ultraviolet light or high voltage electrical discharge. It’s garlicky odor may be discerned after a heavy thunderstorm.
Geometry
The O₂ molecule consists of only two atoms, and two points make a line, right? So what shape is the O₃ molecule? Unlike its more abundant companion, ozone is non-linear. As a result, it carries partial charges on its constituent atoms. See the image.
Now when it comes to visualizing what goes on at the atomic level, there are two common models for ozone. This topic is nicely discussed in the following 1 minute 25 second YouTube video!
Stoichiometry
What is the mechanism for the formation of O₃ in the atmosphere? Granted, ultraviolet light and electrical discharge are two forms of energy. We might be tempted to simply say,
3 O₂ → 2 O₃
However, the real reaction is two-step.
O₂ + λ → 2 O· [high-energy ultraviolet light photolysis]
O· + O₂ → O₃ + kinetic energy
Kinetic energy is heat energy.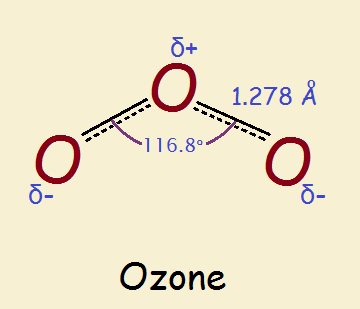
Ozone: the Good, the Bad, the Ugly
Most readers realize high energy UVB ultraviolet light is bad for life. Beach goers coat themselves with protective creams, lotions, and sprays to minimize exposure. Fortunately, there is an ozone “layer” in the lower portion of our stratosphere which blocks much of this energy. Under assault from by-products of technology, preventive efforts seem to be diminishing the damage. The O₃ layer – where it is – is GOOD.
From the word oxygen, we derive the word oxidation. Ozone is a considerably stronger oxidizer than ordinary oxygen. In fact, it is so strong, a variety of organic chemical reaction utilizes O₃ to split a double bond, a feat most oxidizers do not achieve. The simplest instance of this is the reaction of ozone with ethylene gas. See the image. GOOD.
Ozone is no respecter of geography. It is not limited to the atmosphere or the chemistry laboratory. O₃ is produced by electrical discharge. Susceptible component parts of electrical devices can experience oxidation attack. This is familiar to those of us who once owned VCR devices made with rubber parts. BAD.
Ozone production at ground level does occur. For instance, under conditions suitable for it, O₃ in the immediate environment can rise in the vicinity of pine forests. Volatile organic substances related to isoprene combine in the presence of sunlight with natural and manmade pollutants generating haze (often seen along mountain ranges, such as the “Blue Mountains” of Virginia). This haze includes traces of ozone. Again, notice the human element. BAD.
Living tissue, especially tissues possessing mucous membranes such as the lungs are endangered by exposure to ozone, whether acute or chronic exposure. Rubber isn’t the only substance that can be oxidized. ‘These are your lungs on ozone.’ UGLY.
Ozone: In Conclusion
It becomes obvious that O₃ best serves us in our atmosphere. Technology should be designed with O₃ prevention in mind. Nitrogen oxides and other pollutants aggravate matters. Terrestrial ozone should be avoided, especially by those most at risk. Health advisories, if given, should be heeded.
References:
- NOAA – Earth System Research Laboratory: How is Ozone Formed in the Atmosphere?
- Master Organic Chemistry: Alkene Reactions – Ozonolysis
- American Lung Association: Ozone
- EPA: Ozone Pollution
← Back to Food and Health
← Home
