 You almost can’t escape from it. In one form or other, it is nearly everywhere. What is that? Polyurethane. We will focus on polyurethane in furniture refinishing. Then we will discuss its manufacture, and how its manufacture is so dangerous.
You almost can’t escape from it. In one form or other, it is nearly everywhere. What is that? Polyurethane. We will focus on polyurethane in furniture refinishing. Then we will discuss its manufacture, and how its manufacture is so dangerous.
Furniture Refinishing Before Polyurethane
When I was young, I assisted my father in refinishing furniture. First, we’d strip the old finish off. Then, we’d rub the piece with fine very sandpaper. But that wasn’t good enough. So we followed that up by polishing the surface with clean, dry, soap-less steel wool.
Next, we’d apply a choice stain with a cotton rag such as an old torn T-shirt. Once that dried, we’d apply a coating of shellac.
Once that was completely dry, we varnished. However, a coating of varnish produced to shiny an appearance for our taste. To give it a more sophisticated look, we “knocked the edge off” the gloss by rubbing the piece lightly with a slurry what Dad called “rotten stone”. There! We’re done!

That was Then, This is Now
Dad is gone. My furniture refinishing days are through. And polyurethane has largely replaced shellac and traditional varnish. But for the sake of context, what is shellac, what is traditional varnish? Above all, why has this combination largely been replaced by polyurethane?
Shellac
Shellac is a resinous secretion that comes from bugs, specifically lac bugs. It is dissolved in alcohol for use. An alcoholic preparation of shellac has a marvelous odor. The combination of the stain and shellac and stain, when used, convey a richness of color, while nevertheless showcasing the grain of the wood.
Traditional Varnish
Unlike shellac, a varnish had a number of formulations. We used varnish that consisted of a resin, a drying oil, and an organic solvent. Rather than spraying the varnish on, we brushed it on with a quality bristle brush. It is essential to avoid brush strands in the finish.
Advantages of Polyurethane
Polyurethane offers certain advantages that make it popular, not only for furniture, but for gymnasium floor use and other coating applications. It is exceptionally durable. It is resistant to scratches and holds up well under heat. It is produced from industrial chemicals, not requiring starting materials derived from living species.

A Glimpse at the Chemistry
As was the case with traditional varnishes, there are a variety of polyurethanes, using different starting materials, resulting in different finishing properties. We give a simple glimpse of the chemistry involved. This glimpse helps us appreciate why polyurethane manufacture has been and still can be a tricky, dangerous business.
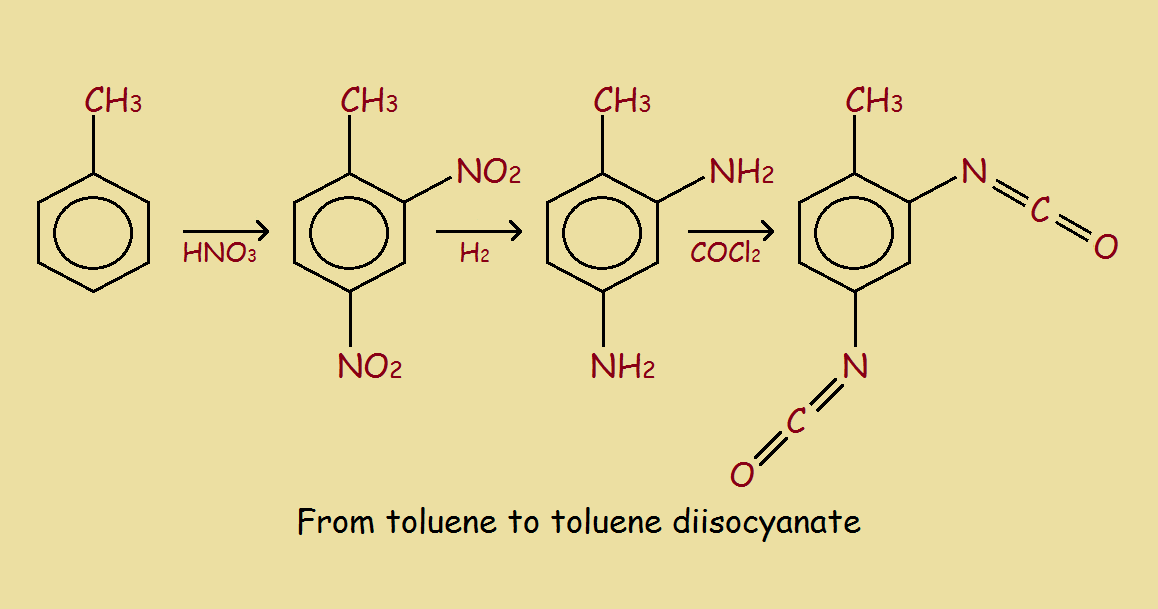
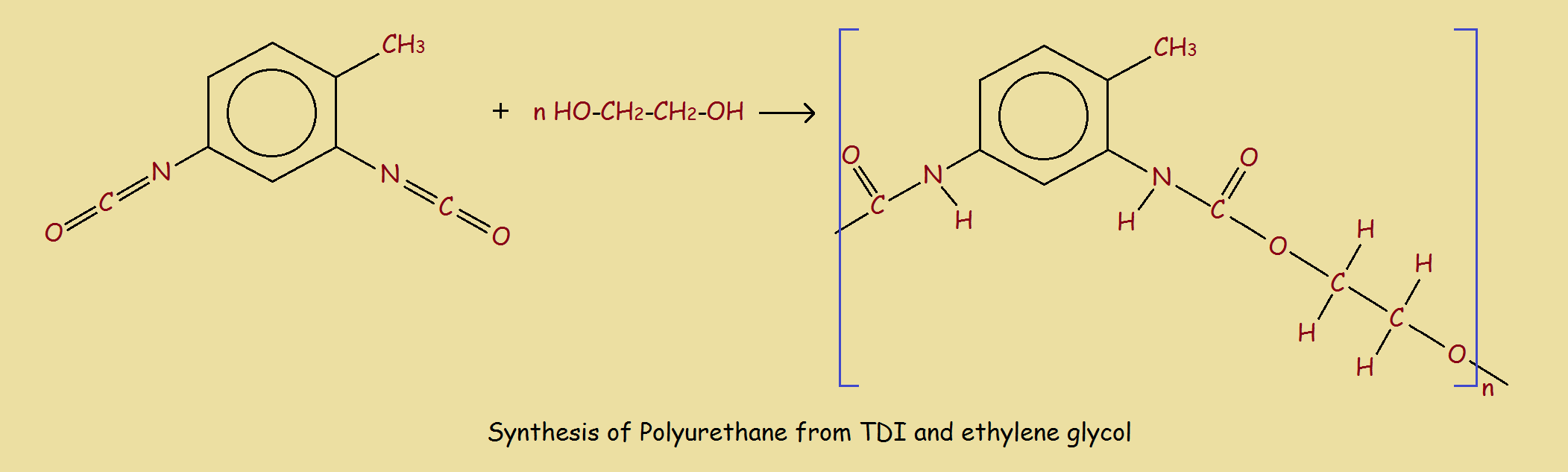
The example we use is the formation of 2,6-toluene diisocyanate, followed by the reaction of the TDI with ethylene glycol to form a polyurethane. The urethane linkages may be written -NH-(C=O)-O-. The reaction schemes are somewhat simplified, but suffice to illustrate the basic chemistry involved. The square brackets with repetition number “n” show polyurethane consists of multiple units. In this instance, the units are two in number, derived from the TDI and the glycol. Hence, this polyurethane is a copolymer.
What are the Dangers?
Among the initial dangers is the use of concentrated nitric acid. The 2,4-dinitrotoluene formed is then reduced (symbolized by the H2) to 2,4-diaminotoluene. A more serious risk is the next step to form the diisocyanate, namely reacting with phosgene! Phosgene (COCl2) is a gas. It is quite poisonous! In fact, it was used in gas warfare during WWI, being responsible for many horrible deaths.
What are the dangers in connection with the diisocyanate itself? That depends. There is no question TDI and similar isocyanates are at least somewhat toxic and need to be handled with caution. However, isocyanates vary in the degree of danger they present. They should not be universally judged by one standard.
For example, TDI is not to be confused with MIC, that is, methyl isocyanate, CH3-N=C=O.
Methyl isocyanate is a gas, a lachrymator (a “tear” gas). It reacts readily with water, generating much heat. It is capable of producing explosions in the presence of water. In 1984, perhaps the worst industrial tragedy to date involved MCI and led to the deaths of thousands in Bhopal India. In time, nearly 20,000 individuals lost their lives!
In Summary
To sum matters up, polyurethanes have arrived and are likely to remain. Purists may prefer the shellac and varnish methods of the past. One does not have to throw away one tool just because another becomes available. Although there are health hazards, they appear to be no worse than for many other industries. Although there has been some outcry that polyurethane is a cancer-causative, there seems to be no clear evidence that such is the case. There is clear evidence, however, that polyurethane, in its many manifestations, is a useful industrial product.
Note: You might also enjoy Disturbing Things in Food
References:
- The Atlantic: Bhopal: The World’s Worst Industrial Disaster, 30 Years Later
- Mortality of workers exposed to toluene diisocyanate in the polyurethane foam industry

Bhopal was a terrible disaster and the company responsible never accepted full responsibility for it!