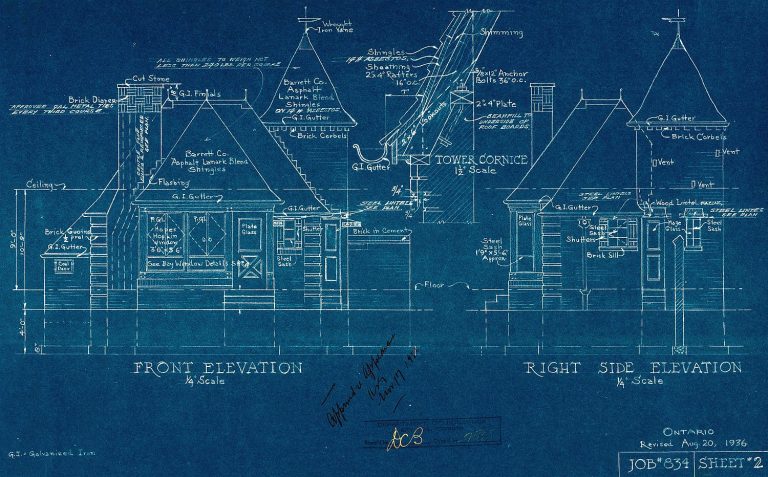
The image you see at right is a traditional architectural blueprint. In fact, this style of blue-inked drawing is how the word blueprint originated. Now the chemistry of this blue colored “ink” is of interest, both historically, and from the science perspective. Let’s see how.
Identifying the Blueprint Ink
The blue ink has a number of names including Paris Blue and Berlin Blue. But the name it is best known by historically is Prussian Blue. Perhaps you will note Prussian Blue is similar to another name, Prussic Acid. Prussic Acid is another name for the deadly poisonous hydrogen cyanide, HCN. And in fact, the ink is closely connected to this acid.
But which was first to be called by a P-word, the acid or the ink?
The Chicken or the Egg?
My first thought was the acid was named first, and the ink named from that. However, it was the other way around! The name Prussic acid “rubbed off” onto the hydrogen cyanide…
What IS Prussian Blue?
Prussian blue was the first historically modern synthetic pigment. A pigment is an insoluble material often used to color paints, inks, or plastics. Prussian Blue is essentially ferric ferrocyanide. Has this identification simplified the discussion, or complicated it?

Introducing: The Ions
First, we will introduce all the element abbreviations we use in this article. H is hydrogen. Fe is ferrum or iron. C is carbon, N is nitrogen. Na is natrium or sodium. Cl is chlorine.
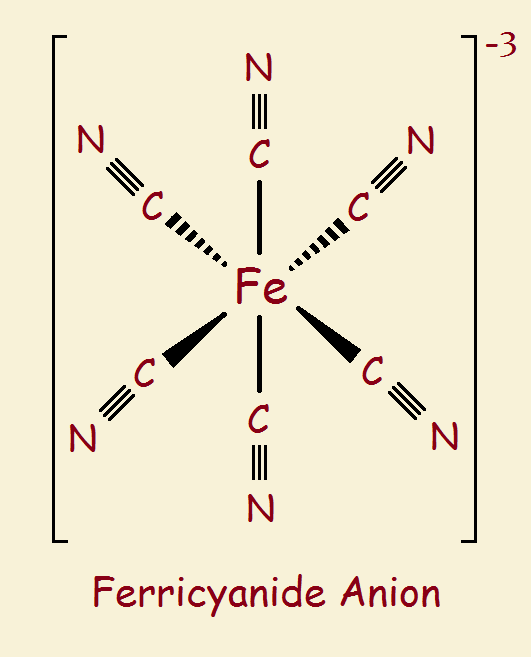
The discussion to follow will involve three simple ions: the ferrous ion (Fe+2), the ferric ion (Fe+3), the cyanide ion (CN-1). These ions are like puzzle pieces used to make puzzles. We introduce two more complicated ions produced by these pieces. They are like clusters of pieces assembled, partially completed puzzles: ferrocyanide and ferricyanide (see the images).
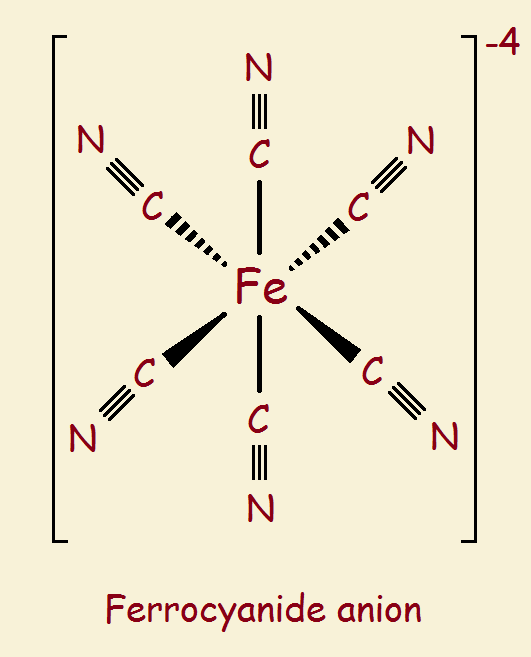
Not everything is shown in the images. In fact, the two images at first glance look identical. What gives one a -3 charge, but the other a -4 charge? It is the iron atom. A ferrous ion is +2. But add six cyanide groups, each a -1, and you get a total of -4. A ferric ion is +3. Add six cyanide groups and you get -3.
Fasten Your Seat Belts
Now the simplest chemical formula for ferric ferrocyanide is written C18Fe7N18. But this tells us nothing about its structure. For something as simple as table salt, sodium chloride, NaCl does the trick. But this is no simple molecule we are discussing. So we some structure hints in our formula. So we write,
Fe+3[Fe+3Fe+2(CN)6]3
The above rendering is accurate and instructive, in contrast with other renderings, which, although concise and accurate, tend to confuse. For example,
Fe4[Fe(CN)6]3
To See the Benefit
To get the full benefit of the structure written out as we have done, see our final image. It strongly suggests a kind of nestling. Once again, it is like building that jigsaw puzzle, now presented as the completed image.
And you thought blueprints were mere drawings, and Prussian Blue was just another color…

References:
- Nature International Journal of Science: Preparation of Ferrous Ferricyanide
- Socratic.org: How to calculate oxidation number of Iron in ferriferrocyanide Fe4[Fe(CN)6]3 ?
← Back to Classic Science
← Home
