
Carbon appears to be unique in its immense chemical diversity. So much so, that an entire branch is devoted to its chemical behavior – organic chemistry. Yet, the name given to this branch of chemistry indicates something of more importance than its being just another branch among many.
Organic chemistry began as the chemistry of living things, the chemistry of all things organic. There are literally millions of carbon compounds incorporated into organic chemistry. The group we will consider here is the saccharides. Some among these consist of multiple saccharide units joined together. Hence, they are termed polysaccharides.
What is a Saccharide?
The word “saccharide” is derived from the Latin sacchararum, “meaning full of sugar.” However, saccharides are not limited to sugars. They include the starches and cellulose.
Saccharides are carbohydrates. The term carbohydrates implies hydrated carbon atoms. A hydrated carbon atoms would consist of one carbon atom, and one water molecule. If a compound consisted of multiple hydrated carbon atoms, it would possess a chemical formula that is, essentially, (CH2O)n. Not a bad approximation.

Rings and Chains of Rings
Sugars of greatest interest are either 5-membered or 6-membered rings. By linking through an oxygen atom,1 single ring sugars (monosaccharides) become disaccharides, even polysaccharides.
And the component sugars do not have to be identical.
For example, sucrose or table sugar consists of a 6-membered sugar, glucose, joined to a 5-membered sugar, fructose (see the image).
Starches
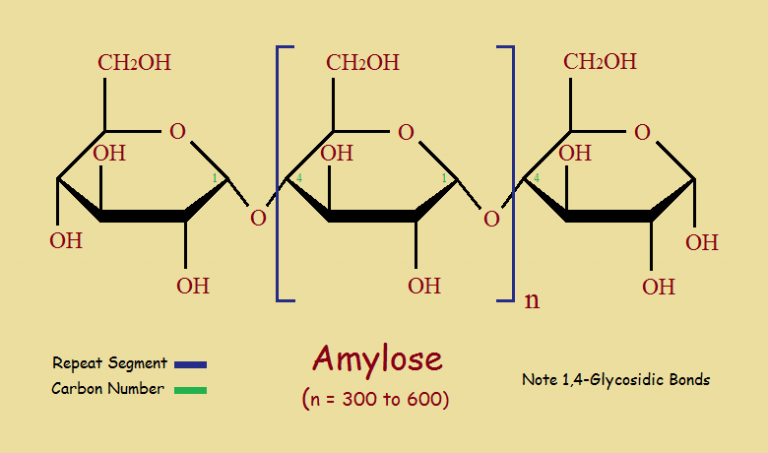
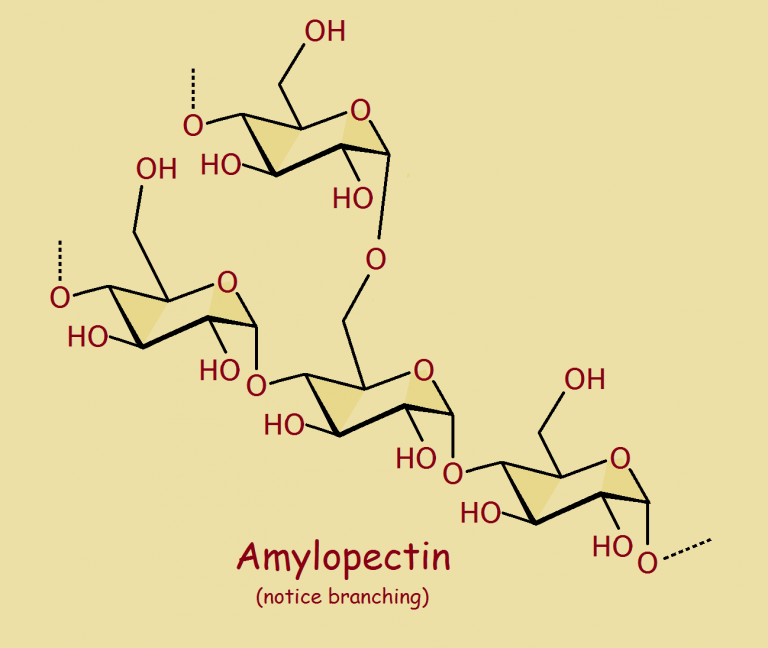
The plant world is put together with a great deal of cellulose, but also starch. And not just the starchy vegetables.
The two basic types of starch are amylose and amylopectin. As might be expected, the two varieties, though related chemically, have different physical properties.
Amylose is built of glucose “blocks”. It has a lower molecular weight than amylopectin and is linear in structure. Thus, amylose more readily crystallizes, and resists digestion more, than the branched amylopectin.
On the other hand, amylopectin readily dissolves in water. Still, both of these substances are essentially polymerized sugar. As starch digests in the body, it releases sugar.
Cellulose
Cellulose is a polysaccharide of very high molecular weight. It is rigid and strong and makes an excellent plant building material. A single molecular fiber of cellulose is illustrated in one of the images. It looks rather like a sugar or starch. Notice the artist’s conception of the rings. The rings are not planar, but assume the approximate form of a lawn chair. This form or conformer is energetically favored.
And There’s More
The more complex image of cellulose shows the relationship between individual strands.
In addition to what we might call “full-blown” chemical bonds, the reader will notice the dashed connections between certain atoms of hydrogen and certain atoms of oxygen.
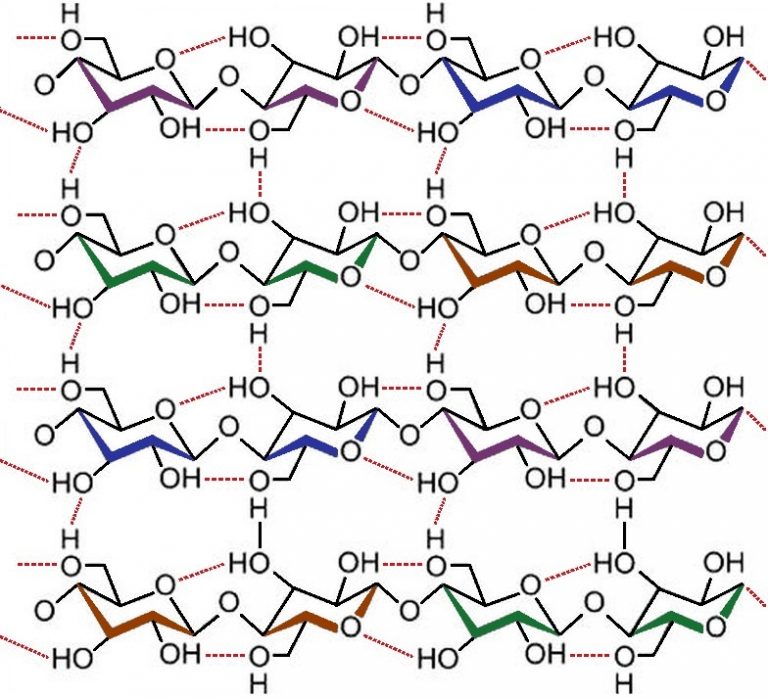
These bonds, called hydrogen bonds, are not as strong. But it is because of their quantity and location that multiple-stranded cellulose is so strong and rigid. This is why makes a tree is so strong. Wood is rich in cellulose.
So How Do Sugars, Starches, and Cellulose Differ?
As the reader may have concluded, sugars, starches, and cellulose vary primarily by molecular weight and by branching.
And in the case of high molecular weight unbranched polysaccharides, the major added factor of hydrogen bonding influences their behavior.
The variety of carbohydrates we call saccharides, are essentially polymers constructed of sugar units, whether of only one variety or of more than one variety. They are joined together by means of oxygen. Their configurations run from straight-chain to branched. The more complex ones can be digested to regenerate their sugar components.
Thus, we conclude, starches, sugars, and cellulose are not so very different at all.
1 Interestingly, note in the image joining together glucose and fructose, how two alcohol groups (–OH) have been united to form an ether linkage (–O–). One could imaginatively think of polysaccharides as polyethers. The official description, however, is that the bond is a glycosidic bond.
Note: You might also enjoy Short, Medium, and Long Grain Rice Vary in Use and Starch Profile
References:
- Purdue University: Carbohydrates
- The Biochemistry Questions Site: Polysaccharides
- The Macromolecules: Polysaccharides
- North Carolina State University: Mini-Encyclopedia of Papermaking Wet-End Chemistry
← Back to Classic Science
← Home

It’s breaking down those linkages into their component sugars that is my downfall in weight loss! Maybe I should start eating cellulose – not digestible!
Some foods contain (or at least contained, past tense) cellulose. It became a real issue for the Public when a well-known Canadian bread included cellulose (they referred to it as sawdust) in their product.