
In 2014, the Korean Journal of Physiology and Pharmacology reported the results of a study on the anti-inflammatory and anti-bacterial properties of shepherd’s purse (Capsella bursa-pastoris).
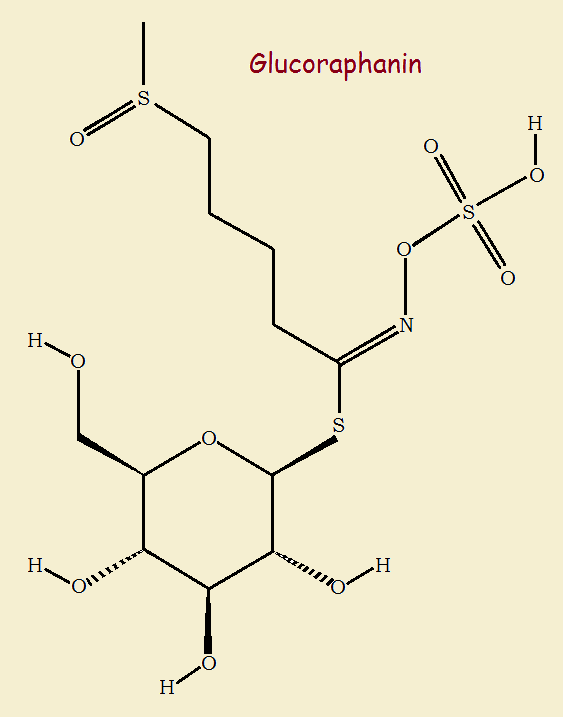
The study spotlighted two compounds formed from the interaction of two other compounds found within the plant, glucoraphanin (a glucosinolate) and the enzyme myrosinase.
When these two substances contact each other, they produce raphanin and sulforaphane.
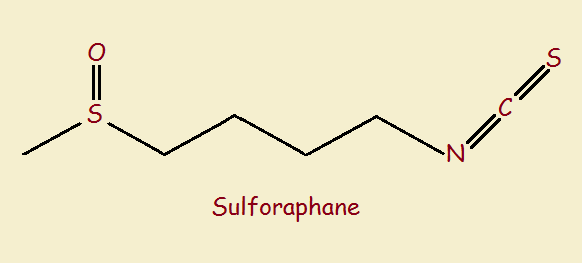 It is the sulforaphane (1-isothiocyanato-4-methylsulfinylbutane) that particularly displays the desired properties. Shepherd’s purse is not alone in producing this interesting substance. Others in the same family (Brassica) include broccoli, cabbage, cauliflower, Brussels’ sprouts, kale, and collard greens. The most common way glucoraphanin and myrosinase are brought together to produce our anti-inflammatory and anti-bacterial is by chewing!
It is the sulforaphane (1-isothiocyanato-4-methylsulfinylbutane) that particularly displays the desired properties. Shepherd’s purse is not alone in producing this interesting substance. Others in the same family (Brassica) include broccoli, cabbage, cauliflower, Brussels’ sprouts, kale, and collard greens. The most common way glucoraphanin and myrosinase are brought together to produce our anti-inflammatory and anti-bacterial is by chewing!
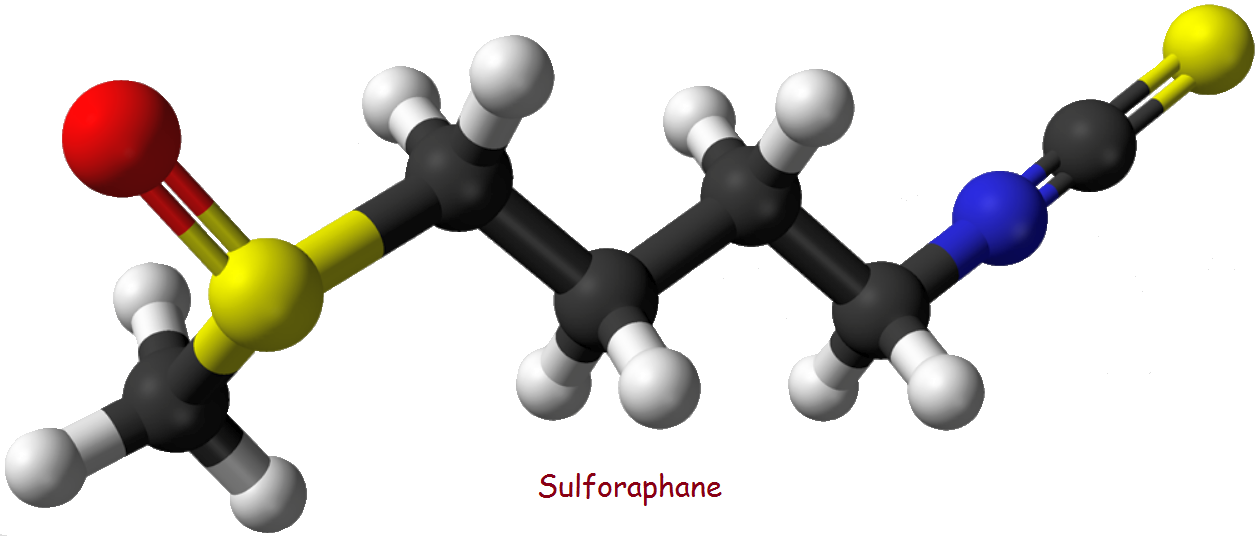
The Structure of Sulforaphane
Most of us are familiar with the poison sodium cyanide, NaCN. That molecule can be oxidized to form sodium cyanate, NaCNO. Change the ordering of the atoms to get sodium isocyanate, NaNCO. Replace the oxygen atoms with an atom of sulfur and you have sodium isothiocyanate.
Sulforaphane incorporates an isothiocyanate like portion that is part of its structure. Still, there is a difference. Sodium isothiocyanate ionizes. In water, the compound breaks apart into positive sodium ions and negative isothiocyanate ions. That is,
NaNCO → Na+ + NCO–
Such is not the case for sulforaphane. It is not ionic.
The Reaction in Greater Detail
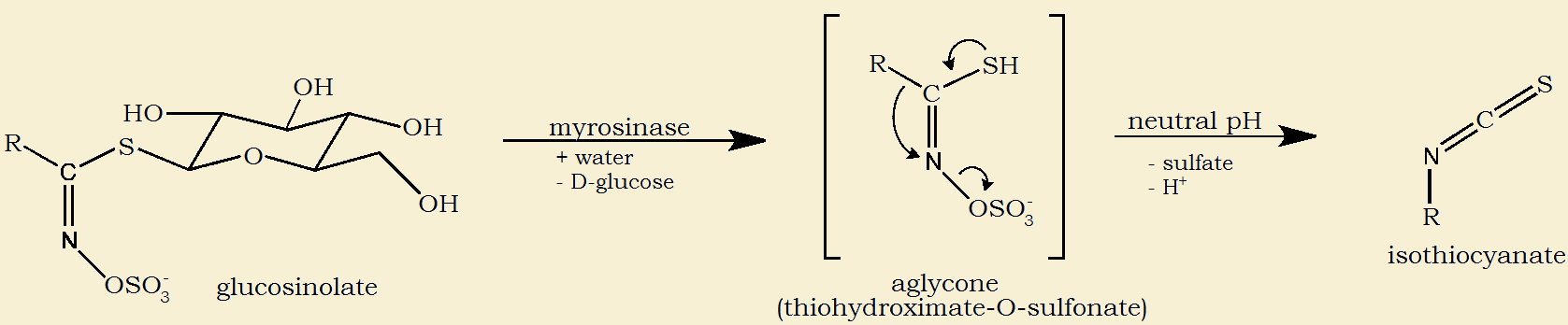
Below are the images and the chemical reactions that ultimately yield our anti-inflammatory. Glucoraphanin, one of many glucosinates, reacts with water and myrosinase to yield D-glucose sugar and an intermediate that, with neutralization, yields raphanin and sulforaphane. Raphanin is found, notably, in the seeds of the red radish, Raphanus sativus.
Concluding Remarks
Clearly the chemistry of even so humble a weed as the shepherd’s purse is not all that simplistic. To reason such complexity is the result of years of coincidental interactions in some slimy ancient puddle (a.k.a. primordial ooze), requires a great deal of faith. More faith, in fact, than does belief in an intelligent creator who is also a master designer.
Note: You might also enjoy What Gives Boiled Eggs Their Sulfur Smell?
References:
