Organic compounds contain carbon and hydrogen, and occasionally other elements. Most notably, these include nitrogen and sulfur, but also phosphorous, chlorine, bromine, and iodine.
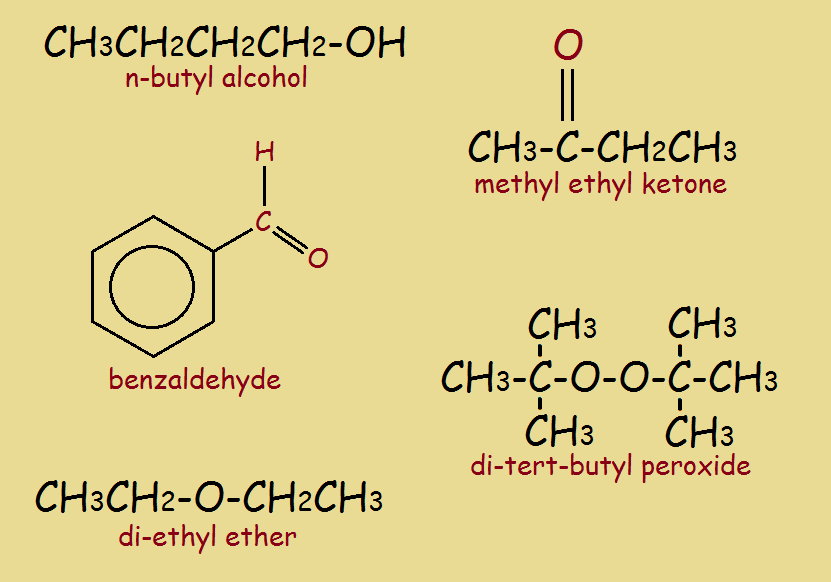
Simple oxygen-containing organics, including n-butyl alcohol, benzaldehyde, methyl ethyl ketone, diethyl ether, and tert-butyl peroxide appear in the illustration at top.
An analog is a structure which is similar to another structure, except that one atom or group is replaced by another (similar-behaving) atom or group. Here, we will discuss sulfur analogs.
Alcohols
The generic structure for a simple hydrocarbon, a compound of hydrogen and carbon, is usually written RH. The equivalent for an aromatic structure is ArH. An alcohol has one hydrogen atom replaced by an –OH group. Hence, an alcohol is written generically, R–OH.
The aromatic counterpart is written Ar–OH. It is called a phenol.
The sulfur analog of an alcohol is called a thiol. Its formula is R–SH or RSH. An alternate name is mercaptan. Butyl thiol, perhaps better known as butyl mercaptan, H3C–CH2–CH2–CH2–SH, written H3C(CH2)2SH for short, is very similar in structure and aroma to some of the components of skunk spray. It is used at very trace levels in household gas to alert homeowners to any gas leak.
Chemistry of Alcohols vs. Thiols
There are some interesting differences between alcohols and thiols. Sulfur-to-hydrogen bonds are longer and weaker than those between oxygen and sulfur. This reduces also the strength of hydrogen bonds between molecules. The reduced cohesiveness between thiol molecules lowers their boiling points in comparison with alcohols. On the other hand, acidity is greater for sulfur analogs.
Aldehydes & Ketones
Aldehydes and ketones both contain a carbonyl group (–C=O or –CO), but there is a slight difference. An aldehyde group comes at a terminal in the chain or a ring, whereas a ketone does not. For instance ethanal (a.k.a. acetaldehyde) is written CH3CHO – whereas CH3COCH3 is dimethyl ketone or acetone.
Generically, an aldehyde is RCHO or ArCHO, while a ketone is R(CO)R‘ or Ar(CO)R or Ar(CO)Ar‘. Notice the –al and –one endings characteristic of aldehydes and ketones, respectively. The primed R, or R’, simply means the two Rs may or may not be identical.
The sulfur analog to an aldehyde or a ketone is a thial or a thione. These are more reactive than their oxy counterparts, especially thials. This may lead, among other things, to the formation of cyclic polymers, such as triamers.
Ethers
An ether is produced when two alcohol and/or phenol molecules, whether different or the same, are joined through the elimination of a molecule of water. For example, two molecules of ethyl alcohol yield one molecule of diethyl ether. The reaction is written,
C2H5OH + HOC2H5 → C2H5–O–C2H5 + H2O
There is a sulfur analog of an ether—a thioether. Older nomenclature labeled these compounds sulfides. Some of these continue to be called sulfides. Thus diethyl sulfide is the same as diethyl thioether, thioethyl ether, and 1,1-thiobisethane. Its formula is,
C2H5–S–C2H5
The physical characteristics of ethers and thioethers are comparable.
Peroxides
An organic peroxide may have any of the following generic formulas:
-
- R–O–O–R’
- Ar–O–O–R’
- Ar–O–O–Ar’
Organic peroxides are often prepared by the oxidation of reactive double bonded hydrocarbons or alkenes. Particularly is this the case for molecules containing allylic or benzylic double bonds.
For instance, 1-methylpropene has a double bond adjacent (allylic) to the methyl group. It is oxidized to the peroxide by the reaction,
(CH3)2C=CH2 + H2O2 → (CH3)2CH–O–O–CH3
Other substances may be added to control reaction specifics.
The sulfur analog of peroxides contains the linkage –S–S– in place of –O–O–. The disulfide (or, more accurately, persulfide) linkage is important in the production of the permanent wave hairdo.
Modifying Sulfur Analogs
Of course, though sulfur is similar to oxygen (for instance, it supports combustion, as in fire and sulfur or brimstone), sulfur has its own personality. Thus some of the analogs can undergo atypical changes. As an example, dimethyl sulfide, which will be recalled as the sulfur analog of dimethyl ether, can be combined with oxygen to form dimethylsulfoxide (DMSO).
Not too long ago dimethyl sulfoxide was introduced as a substance that offered great promise. It can dissolve many substances and in turn itself be dissolved in many solvents.
DMSO penetrates skin tissue to a surprising extent, suggesting its use for topical application of medications. In the chemistry laboratory, DMSO is still of great interest, but early hopes for extensive medical application have been restricted by the U.S. Food and Drug Administration (FDA).
Not Just Analogs
It is thus seen that sulfur analogs of organic compounds containing oxygen are of interest, not only for that reason, but because they can, as was illustrated in the example of dimethyl sulfoxide, undergo unique modifications to produce additional substances useful to science and to commerce.
Note: You might also enjoy Dimethylsulfoxide, DMSO: Snake Oil? Why or Why Not?
References:
- Carey. Organic Chemistry – Chapter 15: Alcohols, Diols, and Thiols . McGraw-Hill
- QuirkyScience. Permanent Wave: Chem-mystery of Curl
- Import Alert 62-06. Dimethyl Sulfoxide (DMSO). U.S. Food & Drug Administration
- Zeyer, et. al.. Oxidation of Dimethyl Sulfide to Dimethyl Sulfoxide by Phototropic Purple Bacteria. National Institutes of Health
