 The fission of one atom of uranium-235 releases 3.24 × 10-11 Joules of energy. One mole of atoms (235 grams) produces 6.022 x 1023 times that amount of energy.¹ How much energy is that?
The fission of one atom of uranium-235 releases 3.24 × 10-11 Joules of energy. One mole of atoms (235 grams) produces 6.022 x 1023 times that amount of energy.¹ How much energy is that?
Energy From Splitting One Mole Uranium-235 Atoms
We calculate,
Eone mole 235U = 3.24 x 10-11 x 6.022 x 1023 = 1.95 x 1013 Joules or 19,500,000,000,000 Joules.
This is a tremendous amount of energy. It is the energy of each mole of uranium found in an atomic bomb! It is millions of times more energy than any chemical reaction.
But is this the ultimate energy matter can produce? By no means!
Total Energy in One U-235 Atom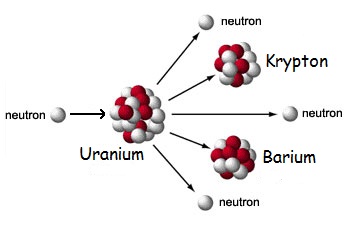
When an atom of uranium is split, it does not entirely disappear. Rather, fragments are produced – atoms of other elements. In our scenario, krypton and barium, plus neutrons, replace uranium. The energy released is by no means the entire amount of energy in the atom.
One atom of 235U has a mass of,
235 grams / 6.022 x 1023 = 3.90 x 10-22 grams
Consuming that one atom produces how much energy? Using Einstein’s first order approximation, E=mc2, we calculate,
E = [3.90 x 10-22 grams][1 kilogram/1000 grams][299.8 x 106 meters/second]2
or,
3.51 x 10-8 Joules
Comparison of One Atom of Each

Thus, we have,
Fission of one 235U atom: 3.24 x 10-11 Joules
Total Energy in one 235U atom: 3.51 x 10-8 Joules
So our uranium atom contains more than 1,000 times the energy produced by merely splitting the atom.
¹ One mole equals one molecular weight (or in this instance, an atomic weight) in grams of a substance. One mole of 235U = 235 grams. It consists of 6.022 x 1023 atoms.
Note: You might also enjoy Do Lone Atoms or Molecules Assume States of Matter?
References:

You know, I never thought before about measuring the difference in mass between the original weight of uranium and the mass of the newly produced products of fission. It’s all very well to say E=MC^2 but when you think about measuring mass and realising that the loss in mass was actually converted to energy, it really brings it home that that famous equation actually means what it says – if you produce energy, you must have lost mass. Wonder if I could fission some of my fat? But that might mean I’d explode!