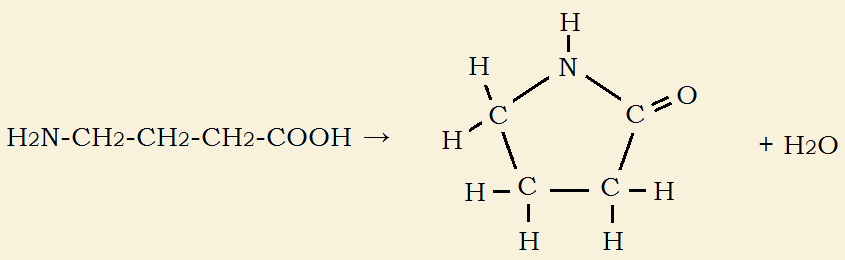 Most have never heard of a lactam. What is it? How can it be produced?
Most have never heard of a lactam. What is it? How can it be produced?
Compounds are chemicals formed by combining two or more different atoms, the basic building blocks of matter. Organic compounds contain carbon, frequently bonded to itself, whether in chains, rings, or some other geometric structure. There are so many organic compounds, they must be categorized according to “functional groups.”
Functional groups are specialized structures within a compound that may contain a special atom, such as nitrogen, sulfur, phosphorous, etc. One kind of compound categorized by functional group is an amide. An amide results when an organic acid group reacts with a nitrogen-containing amine group and loses water.
A lactam is a special kind of amide. It may serve as an intermediate in forming heterocyclic rings.
Inorganic and Organic Compounds
Simple examples of inorganic compounds include NaCl, MgSO4, NH3, and H3PO4. These compounds are, in order, salt, magnesium sulfate, ammonia, and phosphoric acid. The number of different kinds of atoms in each are, again in order, two, three, two, and three. The compound with the greatest number of atoms in this list is H3PO4, with three hydrogen atoms, one phosphorous atom, and four oxygen atoms, or eight atoms complete.
Two examples of organic compounds are CH3CH2CH2OH (propyl alcohol) and C6H6 (benzene). These two substances contain carbon atoms bonded one to another. Ethyl alcohol contains two carbons bonded to each other. Benzene the carbon atoms form a 6-membered ring (See the diagram associated with this article).
Functional Groups
Functional groups are combinations or structures (including double and triple bonds) that are often of greater interest than the overall structure. Examples of functional groups include (along with many others),
-NH2 (amino)
-COOH (carboxylic acid)
-SH (mercaptan)
-C=C- (alkene)
-OH (hydroxyl)
-C=O (carbonyl)
Of these, amides and lactams are combinations of the first two-amino and carboxylic acid.
Amides
An example of amide formation is the reaction between ethylamine and acetic acid with the release of water to form ethyl acetamide.
CH3CH2-NH2 + HOOC-CH3 → CH3CH2-NH-OC-CH3 + HOH
Notice that, in this instance, two short-chain compounds react to form a longer-chain compound.¹
Lactams – Cyclic Amides
Sometimes such a reaction can generate a ring structure containing a similar linkage. Consider the result if two functional groups are a distance apart on the same molecule and react together. For instance, 4-aminobutyric acid, H2N-CH2CH2CH2-COOH. If the correct conditions are applied²
H2N-CH2CH2CH2-COOH → 5-member ring + HOH [see featured image].
The amino group at one end reacts with the carboxylic acid group at the other, closing the molecule to form the ring. This kind of ring is called a lactam. The type of lactam is designated by using a Greek letter prefix that indicating the number of carbons in the ring, not counting the carbonyl group.
For instance, if there are two such carbons, the prefix is beta (the 2nd letter of the alphabet) – if there are four, the prefix is delta, and so on. The ring formed in the above reaction is a gamma-lactam. It’s name is based on the number of carbon atoms in the skeleton-in this instance, butyrolactam.
There are other ways of naming the structure, one of which is 2-pyrollidinone.
Reducing the Carbonyl in Lactam to CH2
The carbonyl of a lactam can be reduced to produce a heterocyclic ring. Heterocyclic refers to the presence of a non-carbon atom in the ring. It is thus easy to produce a four member ring containing a nitrogen atom, or a five member ring, a six member ring, and so on.
Such rings often occur in very important organic compounds related to living things. There are heterocyclic rings in the active ingredients of a plethora of medications. There are a variety of methodologies for reducing the carbonyl. To visualize the end result, again refer to the image associated with this article.
1 Although it isn’t obvious as drawn, the carbon-containing chain formed consists of five atoms, C-C-C-N-C-C. The oxygen is actually attached to the next-to-last carbon by a double-bond off to the side.
2 Utilizing different conditions can produce a polymeric product. The amino group of one molecule can react with the carboxylic acid of second molecule, which adjoins to a third molecule, and so on.
Note: You might also enjoy reading Epoxide Ring Preparationby Oxidation of Alkenes
References:
- The Amide Linkage: Structural Significance in Chemistry, Biochemistry, Materials Science, by A. Greenberg, et. al.
← Back to Classic Science
← Home
