
Acid-Base and Redox: When they can, chemists categorize chemical reactions into types. Some specialized varieties of chemical reaction include those named for specific individuals involved in their discovery.
As examples, there are the Diels-Alder reaction, the Claisen rearrangement, the Dieckmann condensation, and the Friedel-Crafts alkylation.
Yet, many chemical reactions can be classified simply as acid-base reactions, or as oxidation-reduction (REDOX) reactions. Consider examples of each…
Acid-Base Reactions
When I was young, I was deeply interested in rocketry. My parents bought me one of those red plastic rockets that included a pump. You partly filled the rocket with water, then pumped air into it until the pressure made it difficult to pump more. Then you aimed it upward and pushed a button. SWOOSH! Up it sailed, perhaps 50 feet into the air. Perhaps more.

But I had to be different. So I put some baking soda inside and poured vinegar in and pumped. The pressure was much, much greater. When I pushed the button, the rocket shot upwards. What it then did, I didn’t have a clue. I never saw it again. The extra driving force was due to the production of carbon dioxide gas. That is what increased the pressure so that the rocket did what it did. The chemical reaction between the baking soda and vinegar — an acid-base reaction — was…
CH3COOH + NaHCO3 → CH3COONa + CO2↑ + H2O
Vinegar is a 5% solution of acetic acid. Baking soda (sodium bicarbonate) acts as a base. Mix these two together in a dish, and you get bubbling. In my case, those bubbles were trapped in the rocket.
Note that in an acid-base reaction, there is typically the transfer of one or more atoms of hydrogen.1 In this instance, the transfer produces a molecule of water.
Sodium Bicarbonate From a Different Vantage Point
Our second example was chosen because it is a curious example of how some compounds can act as both an acid and a base! We once again consider sodium bicarbonate.
NaOH + NaHCO3 → Na2CO3+ H2↑
Why does the bicarbonate act in the first instance as a base and in the second instance as an acid? Because acidity and basicity are relative. Sodium bicarbonate is basic when compared with acetic acid. However, it is acidic when compared to sodium hydroxide.
Oxidation-Reduction Reactions
Oxidation and reduction involves the transfer of electrons during a chemical reaction. The loss of electrons is oxidation. A piece of iron oxidizes when it rusts, the metal losing electrons when it forms the hydrated complex of the oxide
Fe3O4
We’ll consider three REDOX examples. For instance, a concentrated ferric chloride solution can be used to etch the copper of circuit boards. At its most basic level, the chemistry is,
FeCl3 + Cu → FeCl2 + CuCl
In the above reaction, the ferric (Iron III) ion is reduced to ferrous (Iron II), whereas the copper atom is oxidized to a cuprous ion. How is this so? Because oxidation involves the loss of electrons and reduction involves the gain of electrons.
Fe+3 + e– → Fe+2
Cu – e– → Cu+
Our Second REDOX Example
Now we will consider the oxidation of isopropyl (rubbing) alcohol utilizing potassium dichromate. We write,
2 H3C-CH(OH)-CH3 + K2Cr2O7 + H2SO4 → 2 H3C-(C=O)-CH3 + Cr2O3 + K2SO4 + 3H2O
In this instance, the isopropyl alcohol (written more simply C3H7OH) is oxidized to dimethyl ketone or acetone
(CH3)2=CO
The dichromate negative ion, or anion, (Cr2O7)-2 (or Cr+6) is reduced to Cr+3.
Our Third REDOX Example
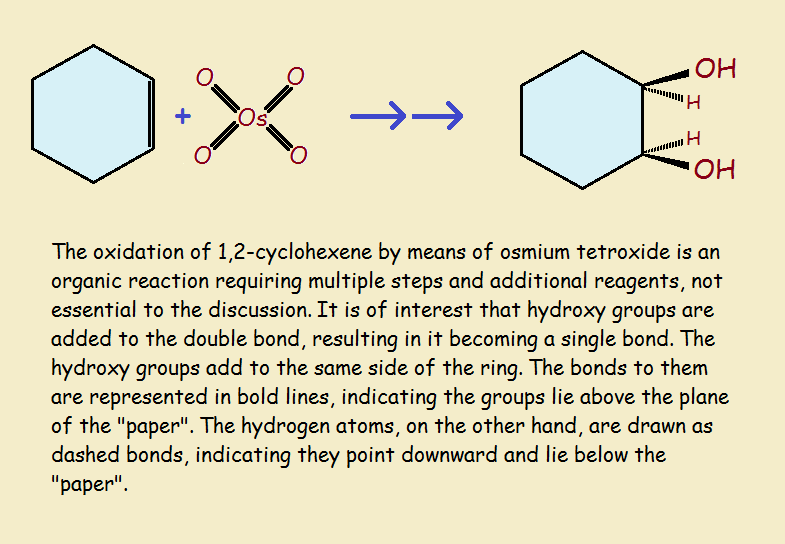
For our third and final example of oxidation-reduction reactions, we consider the oxidation of cyclohexene by osmium tetroxide. See the illustration for convenient visualization.
The double bond compared to an otherwise identical single bond is in a higher oxidation state. So it is oxidized by the reaction. Osmium tetroxide resides in an unusually high level of oxidation, a +8 oxidation state! It is, needless to say, reduced by the reaction sequence.
1 We will avoid the topic of Lewis acids, which makes our discussion needlessly complex and not very helpful.
Note: You might also enjoy Introduction to Chemistry Subscripts and Superscripts
References:
- Master Organic Chemistry: Reagent Friday: OsO₄ (Osmium Tetroxide)
- Journal of Chemical Education: Oxidation of isopropyl alcohol to acetone. A student experiment
