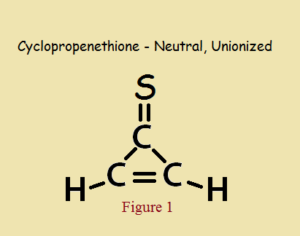
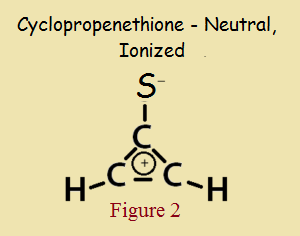
As small as it is, and even with a heteroatom in its make up, cyclopropenethione is aromatic, in the same way cyclopropenone is aromatic. Its aromaticity is not due to a theoretically electrically neutral structure, as in Figure 1, but to its “alternative” zwitterionic structure, shown in Figure 2.
Aromatic Characteristics
Hückel descriptors fall short of aromaticity if cyclopropenethione exhibited only the structure drawn in Figure 1.
In addition to a closed and flat ring, aromaticity requires a 4n+2 number of π-electrons (pi), where n is usually a small integer. In the un-ionized form of Figure 1, each carbon atom has a π-electron, for a total of 3 electrons. This is because every double bond consists of one π-electron plus one σ-electron (sigma) per constituent atom.
Widening Perspectives: Cyclopropenethione
The examples of cyclopropenone and cyclopropenethione suggest it might be wise to widen our perspectives as to what molecular species might prove to be aromatic.
In fact, I have myself meditated on that possibility, though in doing so perhaps I open myself to criticism. I wrote of it here: Are Additional Varieties of Aromaticity Achievable?
References: