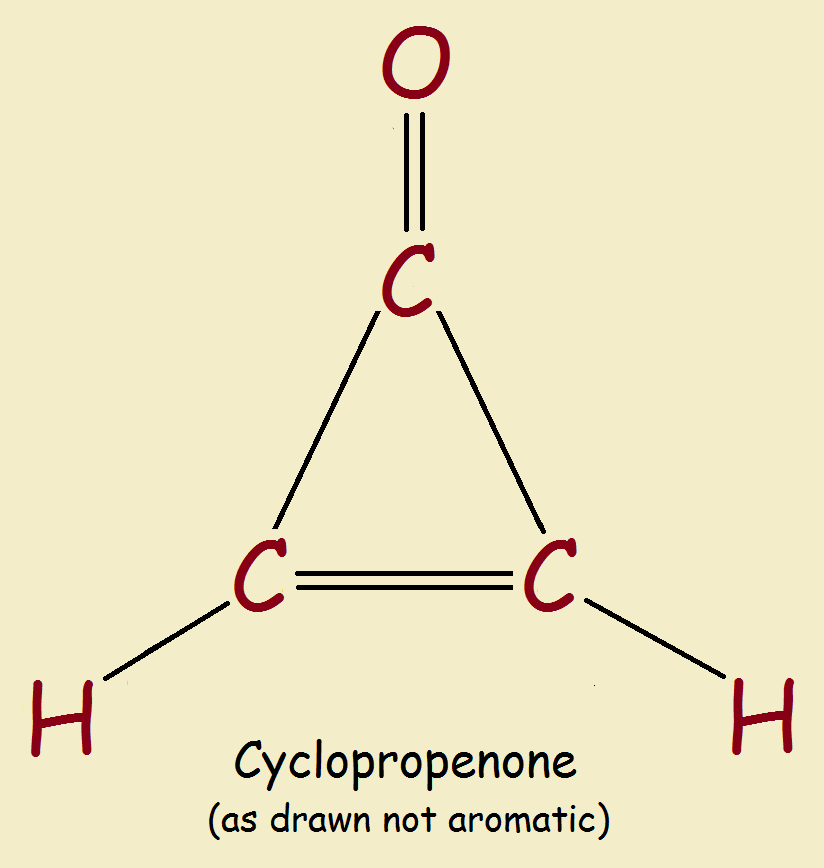
 Is cyclopropenone aromatic? Before reading on, try to anticipate the answer to that question.
Is cyclopropenone aromatic? Before reading on, try to anticipate the answer to that question.
When most students think of aromatic, they think of simple hydrocarbons—compounds composed of hydrogen and carbon atoms only. The best known example of an aromatic hydrocarbon is the 6-sided cyclic compound benzene—C₆H₆.
Benzene, if drawn according to classical structures, has alternating single and double bonds between carbon atoms.
Basic Qualifications for Aromaticity
A double bond consists of one sigma (σ) and one pi (π) bond. If the ring is single rather than multiple (as, for instance, for naphthalene, which is comprised of two fused rings), and if it has 4n+2 completely conjugated π electrons in a closed loop or ring, is neutral, and is flat, the structure obeys Hückel’s rule and will be aromatic. There are non-neutral species, however, that are also aromatic, such as the tropylium cation, the cyclopentadienyl anion, and the cyclopropenyl cation.
Charged Aromatic Structures
The tropylium cation is also known as the cycloheptatrienyl cation. It has, not seven π electrons, but because it is a cation, six.
The cyclopentadienyl anion has, because it bears a negative charge, has not five π bonds, but six.
The cyclopropenyl cation is the smallest of the aromatic structures. It has, not three π bonds, but two, because it, too, is a cation, missing an electron.
Perhaps you would enjoy this Khan Academy video that includes cyclopropenone in its discussion.
Cyclopropenone Aromatic? Heteroatoms
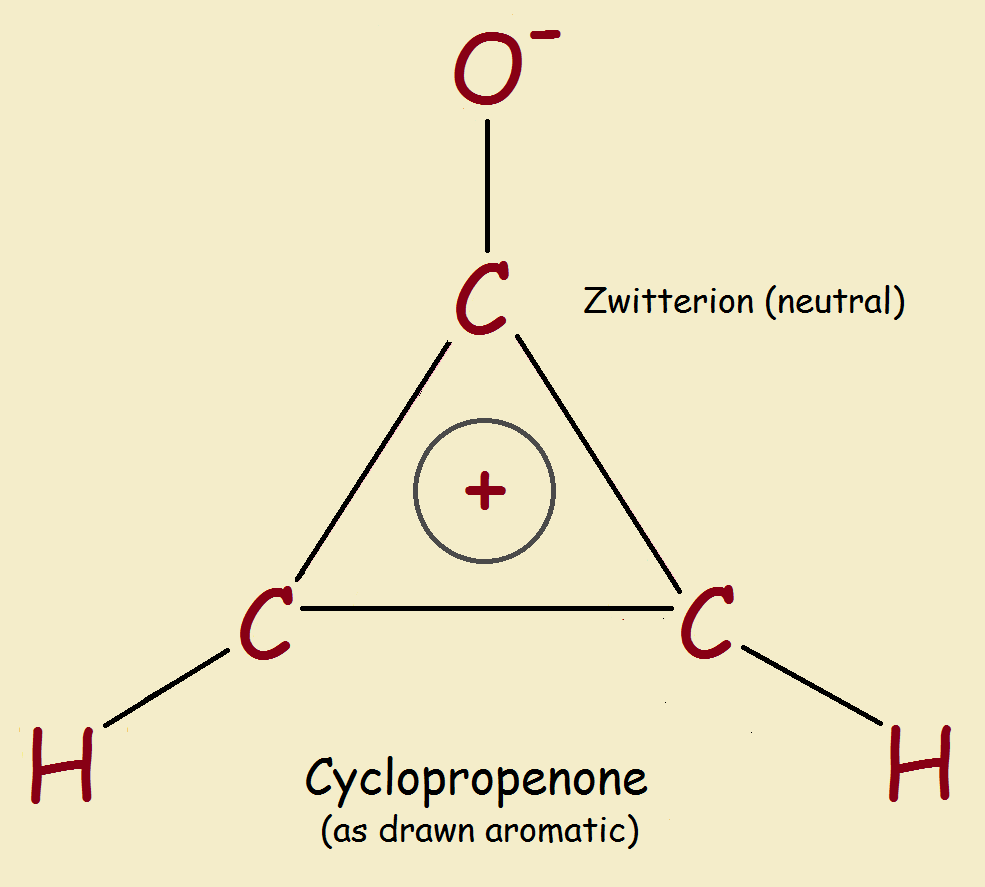 There are, in addition, aromatic species that contain a heteroatom such as nitrogen or oxygen, and are aromatic just the same. The smallest neutral molecule considered potentially aromatic, is cyclopropenone. Is this molecule aromatic? Speculations have existed for some time concerning this tiny ring structure. It appears it is, though naturally, characteristics such as ring strain would weaken the structure’s stability.
There are, in addition, aromatic species that contain a heteroatom such as nitrogen or oxygen, and are aromatic just the same. The smallest neutral molecule considered potentially aromatic, is cyclopropenone. Is this molecule aromatic? Speculations have existed for some time concerning this tiny ring structure. It appears it is, though naturally, characteristics such as ring strain would weaken the structure’s stability.
Note: You might also enjoy Hückel’s Smallest: Aromatic Cyclopropenyl Cation
References:
- ACS: C&EN archives: Experiments show cyclopropenone is aromatic
- NPTEL: Benzene and Related Compounds: Aromaticity
← Back to Classic Science
← Home

Short, sweet and accurate as always – and aromatic too!
So basically nobody knows (yet)?
Well, it’s a moot point. You will note the ACS reference. The ACS is the American Chemical Society. It suggests empirical studies point to aromaticity.
The double-ion structure, though certainly not the most desirable of features, is somehow intuitive for me. If the 3-membered ring has a double bond, it just seems natural it would not be as stable strain-wise as a ring with all the bonds of equal length. A kind of equilateral ring. So though ring strain tends to fight against energy stabilization due to aromaticity, in this case it suggests to me it assists the process.