
If you’re like me, you enjoy not only some of the more modern offerings of the motion picture industry, but many of the gems from the golden era. Sadly, some excellent productions are either damaged or lost due to nitrocellulose motion picture film decomposition.
Nitrocellulose Motion Picture Film
Motion picture films incorporate an emulsion atop a “plastic” substrate. Historically, this substrate has been made from three basic materials. The oldest of film varieties was cellulose nitrate, a.k.a. nitrocellulose. Films produced using this material were, and are, the most susceptible to loss through deterioration. It’s a matter of chemistry.
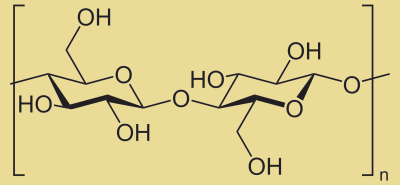
Chemical Structures
Notice the structure of cellulose, a major component of plant fiber. Then examine the largely similar structure of nitrocellulose. Take note of the differences. Yes, they are identical, except the hydroxyl groups (-OH) are changed into nitrate groups (-NO3)+. This is accomplished by reacting cellulose with nitric acid in the presence of a dehydrating agent (such as concentrated sulfuric acid). Perhaps it is easiest to envision the process,
Ring-OH + HNO3 → Ring-O-NO2 + H2O
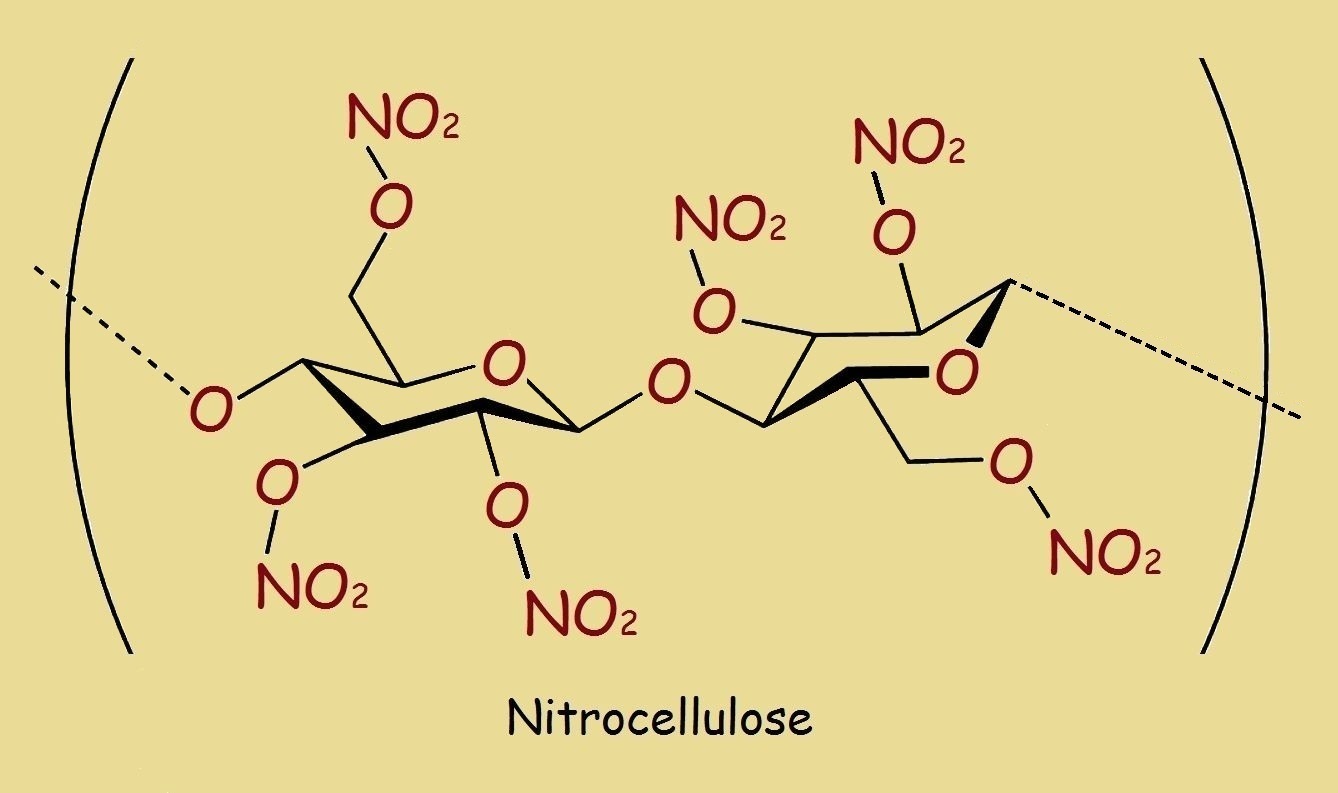
Decomposition and Deterioration
The problem, as previously mentioned, is the chemical deterioration or breakdown of nitrocellulose. Cellulose nitrate is used in a variety of ways. It is not used solely as film. Another use, for example, was in lacquers. Heat and ultraviolet radiation hasten decomposition through reaction such as the following,
Ring-O-NO2 → Ring-O• + •NO2
In addition, typically during its manufacture only about 29 out of every 30 hydroxyl groups are replaced by nitrate groups. The thirtieth group is replaced by a variety of sulfate group. For example, the formation of cellulose hydrogen sulfate,
Ring-OH + H2SO4 → Ring-OSO2OH + H2O
Alternately, some cellulose rings might react with the sulfuric acid dehydrating agent to produce dicellulose sulfate. That reaction can be written,
Ring-OH + H2SO4 + Ring-OH → Ring-OSO2O-Ring + 2 H2O
The hydrogen sulfate listed first is seriously hygroscopic (that is, it absorbs water from the surrounding air), leading to decomposition via de-esterification (loss of the sulfate or nitrate group).
Note: You might also enjoy What is the Origin of Races?
References:
