Aromatics are separate category of organic compounds. Often aromatic compounds are electrically neutral. However, a few do carry a charge. They are ions.¹ In fact many aromatic ions would not be aromatic if they lost their charge. We will consider the aromatic tropylium cation in this article.
Three Carbon Atoms
The smallest aromatic ion is the cyclopropenyl cation. The neutral cyclopropene molecule possesses 4 π-electrons. That is not an aromatic number. By making it a cation, it loses 2 of them, meeting the qualification for aromatic compounds.¹
Four Carbon Atoms
On the basis of π-electron count alone, one might suppose a cyclobutadienyl di-anion or a cyclobutadienyl di-cation, would display aromaticity. That is not the case. There are other requirements to be aromatic.²
Five Carbon Atoms
Cyclopentadiene is the next hydrocarbon that can produce an aromatic ion. This ion is negative. It is the cyclopentadienyl anion (C₅H₅⁻).³
Six Carbon Atoms
After that five-carbon ring comes the six-member benzene ring, which is both aromatic and neutral. It is the quintessential aromatic compound. It is the most commonly recognized aromatic.
Seven Carbon Atoms
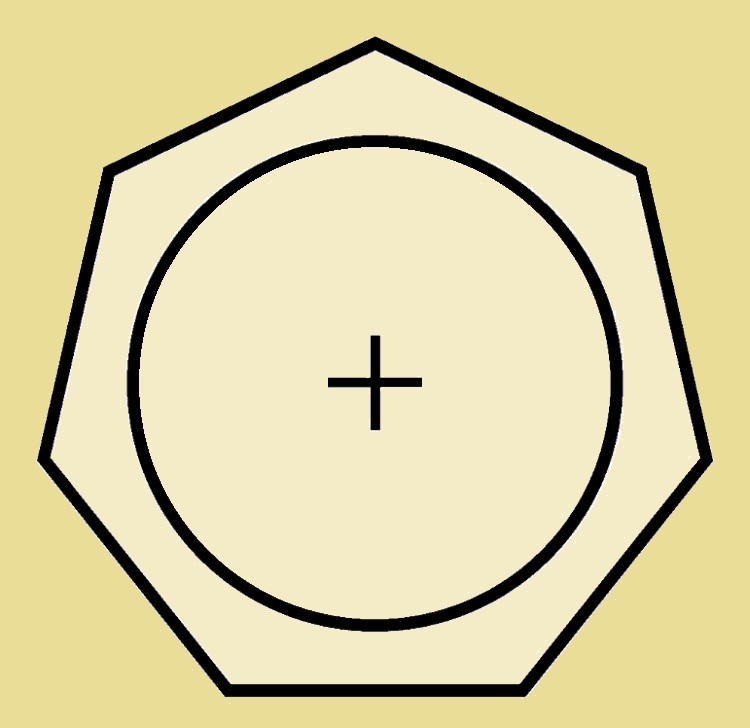
At last we reach the seven-carbon atom cyclic compound, cycloheptatriene. This species has a slight pucker at the completely σ-bonded carbon atom. By changing it to the cation (C₇H₇⁺), we have an appropriate 6 π-electrons and an at least nearly planar structure. And the positive charge is not localized on just the one carbon. Thus the cycloheptatrienyl cation is both stable and aromatic.
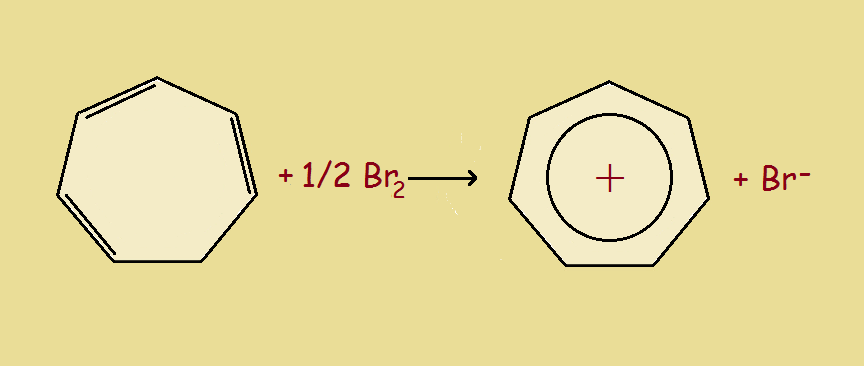
Aromatic Tropylium Cation
The cycloheptatrienyl cation is also called the aromatic tropylium ion. Where does it get the common name tropylium? From the compound tropine, which in turn derives its name from atropine. Both tropine and atropine contain a 7-carbon atom ring.
1 The Hückel Rule for aromaticity says a compound must have 4n + 2 π-electrons. It doesn’t say the compound must be electrically neutral.
2 Among them: the structure must be cyclic and the ring must be flat, there must be sufficient p-orbital overlap of contributing atoms, and delocalization must lower the electronic energy.
3 See The Aromatic Cyclopentadienyl Cation, by the author.
Note: You might also enjoy Azulene or Cycloheptatrienyl Cation Cyclopentadienyl Anion “Salt”?
References:
- UC – Davis: Aromatic Ions and Antiaromaticity
- Western Washington University: Aromaticity and Hückel’s Rule
← Back to Classic Science
← Home
