Is it possible that for an ion to be aromatic? Yes. Consider the aromatic cyclopentadienyl anion.
Hückel’s Rule dictates a flat ring with 4n + 2 π (pi) conjugated electrons. The smallest neutral ring with these qualifications has n = 1. It is benzene (C₆H₆). But Hückel’s law does not require an electronically neutral structure.
The smallest aromatic ion is the cyclopropenyl cation¹ (C₃H₃⁺). Is there a negative ion that has 6 π electrons and is aromatic? The answer is yes. The aromatic cyclopentadienyl anion (C₅H₅⁻).
Neutral Cyclopentadiene
Neutral cyclopentadiene is flat. It is pentagon shaped. Three of the carbon-carbon bonds are single bonds. The other two are double bonds. At each of the four ends of the two double bonds there is one hydrogen atom.
The one carbon atom with two carbon-carbon single bonds has two hydrogen atoms. Remove one positive hydrogen ion, and the ring is left with a negative charge. It includes an electron pair that adds the + 2 in the 4n + 2 aromaticity equation. Cyclopentadiene loses that one H⁺ readily. This is the same as saying cyclopentadiene is acidic. The departing hydrogen ion is a good “leaving group.”
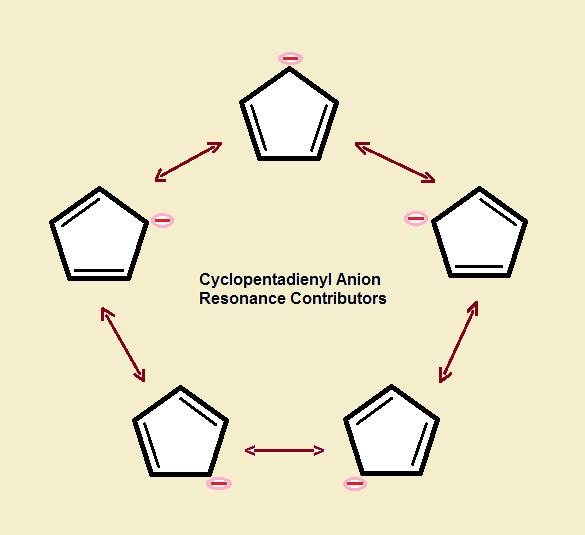
Equivalence of Carbon-Carbon Bonds
Double bonds are shorter and more reactive than single bonds. Yet, when cyclopentadiene loses H⁺, all the bonds of the ring change. Each C-C bond becomes equal in strength. So each is equal in length. Each is shorter than a single bond yet longer than a double bond.
Every one of the C-C bonds possesses π character. A ring current is present. All of this shouts: aromatic!
The Aromatic Cyclopentadienyl Anion
The increased stability from aromaticity pushes the chemistry of the cyclopentadienyl anion toward substitution rather than addition reactions.
¹ A cation is a molecule in which one or more atoms is exhibits a full positive charge.
Note: You might also enjoy…
- The Aromatic Tropylium Ion (Cycloheptatrienyl Cation)
- Azulene or Cycloheptatrienyl Cation Cyclopentadienyl Anion “Salt”?
References:
- UCLA: Illustrated Glossary of Organic Chemistry: Hückel’s Rule (4n + 2 rule)
- UC-Davis: Hückel’s Rule
← Back to Classic Science
← Home

Sir, in the compound furan, only one lone pair of electrons of oxygen is in [the] resonance [structure]. Why not both pairs?
Chemical structures seek to attain the lowest energy state they can attain. For furan, the aromatic structure possesses a lower energy. The 4n+2 pi-bond-plus-electron-pair number calls for only the one pair of electrons. For visual impact, see: https://socratic.org/questions/why-is-furan-an-aromatic-compound