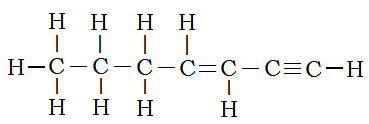Alkanes, alkenes, and alkynes are made up of carbon and hydrogen. The carbon atoms in each exhibit a valency of four. Though these three hydrocarbon varieties are similar, there is a difference in bonding. We illustrate this with the simplest example: ethane vs. ethene vs. ethyne.
Common and Scientific Names
Common names are names given to many compounds, but they may mislead the uninitiated. For instance, the scientific names of alkenes contain the suffix –en(e) as part of their name. But acetylene is not an alkene. It is an alkyne. The scientific names of alkynes contains the suffix –yne. Acetylene is scientifically named ethyne. Yet, it nomenclature is not the only difference between alkanes, alkenes, and alkynes.
Alkanes
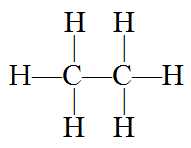
Alkanes are completely saturated compounds. That means every carbon atom bonds to four other atoms. Ethene has two carbon atoms and six hydrogen atoms. Why not eight hydrogen atoms? Because the two carbon atoms bond to each other, using up one bond of each carbon atom, resulting in 8 – 2 = 6 remaining. Thus ethane’s formula is written:
Alkenes
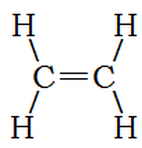
Ethene is much like ethane, except instead of a single bond between carbon atoms, it has a double bond. This uses two of the four bonds from each carbon atom, leaving a total of 8 – 4 = 4 bonds to attach to hydrogen atoms. We write ethene’s formula:
Alkynes
Ethyne has a triple bond between the two carbon atoms, leaving 8 – 6 = 2 bonds to attach to hydrogen atoms. Ethyne is written:
Combinations
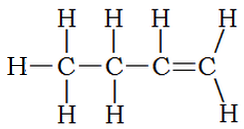
What about compounds that contain a number of single bonds and one double bond? Although the majority of the compound resembles an alkane, it is classified as an alkene. Thus the following structure is named 1-butene:
Alkanes, Alkenes, and Alkynes – Pecking Order
When it comes to naming organic compounds, reference is made to various rings, pendant groups, and bonding combinations. It follows a definite pecking order. For full details, visit the International Union of Pure and Applied Chemistry, or IUPAC.
For only a combination of single, double, and triple carbon-carbon bonded simple alkanes, the order is triple bonds take precedence over double bonds, which, in turn, take precedence over single bonds. Thus the structure:
is hept-3-en-1-yne. The chain is seven carbons long, so the root is “hept”. The double bond lies between carbons 3 and 4. The lower number is used to specify location. The triple bond is between carbons 1 and 2.
Types of Bond
It ought to be pointed out that a double bond is not equal to two single bonds. Neither is a triple bond the equivalent of three single bonds. A single bond is inline and is called more formally a sigma bond, whereas a double bond consists of one sigma and one pi bond. A triple bond consists of one sigma (σ) and two pi (π) bonds. The pi bond greatly influences reactivity and reaction behavior.
Note: You might also enjoy Straight Chain Alkanes: Predicting Properties
← Back to Classic Science
← Home