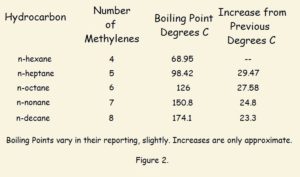
 Straight chain alkanes are compounds with a non-branched backbone of carbon atoms. In addition, as many hydrogen atoms as possible are attached to each carbon atom. Straight chain alkanes are “saturated” hydrocarbons, which means they are completely filled up with hydrogen. See Figure 1 for examples of straight chain, branched chain, saturated, and unsaturated hydrocarbons.
Straight chain alkanes are compounds with a non-branched backbone of carbon atoms. In addition, as many hydrogen atoms as possible are attached to each carbon atom. Straight chain alkanes are “saturated” hydrocarbons, which means they are completely filled up with hydrogen. See Figure 1 for examples of straight chain, branched chain, saturated, and unsaturated hydrocarbons.
Methylene
With the exceptions of methane and ethane, straight chain alkanes differ in the number of methylene groups (CH₂) each contains. Thus the first five hydrocarbons, methane, ethane, propane, butane, and pentane are written,
CH₄
CH₃CH₃
CH₃CH₂CH₃
CH₃CH₂CH₂CH₃
CH₃CH₂CH₂CH₂CH₃
Another method of writing straight chain alkane chemical formulas for the butane and pentane structures above is,
CH₃(CH₂)₂CH₃
CH₃(CH₂)₃CH₃
General Formula
From this, we can formulate the rule for the formula of a straight chain alkane is,
CH₃(CH₂)nCH₃ where n = 0, 1, 2, 3, …, ∞
The orderly increase in methylene groups of 0, 1, 2, and 3, drawn above, suggests physical properties should change in an orderly fashion as the number of methylene groups is changed. Is this the case? Consider the property of boiling point.
Boiling Point
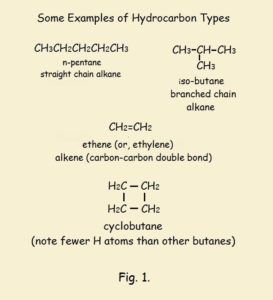
Logically, the fairest test of property change with increasing methylene groups should occur in alkanes whose structures contain a high percentage of methylene groups in comparison to the end methyl groups (CH₃). Therefore, we will consider the boiling points of the liquid straight chain alkanes n-hexane, n-heptane, n-octane, n-nonane, and n-decane.¹
Boiling Point –vs– Number of Methylene Groups
Figure 2 gives approximate data for the changes in the boiling points of the above listed straight chain hydrocarbons. Clearly, as the percent of methylene groups increases, the boiling point lowers by 20⁺ degrees, Celsius. However, with increasing carbon number, the decrease is incrementally less. That is, the changes of the changes of the boiling points (comparable to the mathematical concept of a 2nd derivative) becomes less and less.
Straight Chain Alkanes – Other Properties
Although we have only considered boiling points, other physical properties change predictably, using similar reasonings. So it is that the chemist seeks to establish order for the data found in his chemical pursuits.
1 The prefix n- before each hydrocarbon name means normal, or straight chain. The prefix is important. For instance, there is not one, but two chain butanes. The branched isobutane is seen in the image associated with this article. As the number of carbon atoms increases, the number of possible structures increases. There are also cyclic isomers, such as cyclobutane, not considered here.
Note: You might also enjoy Tetra-Tert-Butyl Methane – The Acyclic Alkane that Seemingly Should Exist
References:
← Back to Classic Science
← Home