 Many moons ago, chemistry was divided into two large groups, organic chemistry and inorganic chemistry. Organic chemistry was thought to be the ‘chemistry of life’. This was because a mysterious “vital force” associated with life was thought essential to produce organic compounds.
Many moons ago, chemistry was divided into two large groups, organic chemistry and inorganic chemistry. Organic chemistry was thought to be the ‘chemistry of life’. This was because a mysterious “vital force” associated with life was thought essential to produce organic compounds.
Most organic compounds consist of carbon and hydrogen atoms, with or without nitrogen, oxygen, phosphorous, or other atoms. We will discuss a simple hydrocarbon. A hydrocarbon consists solely of carbon and hydrogen.
Hydrocarbon Bonds
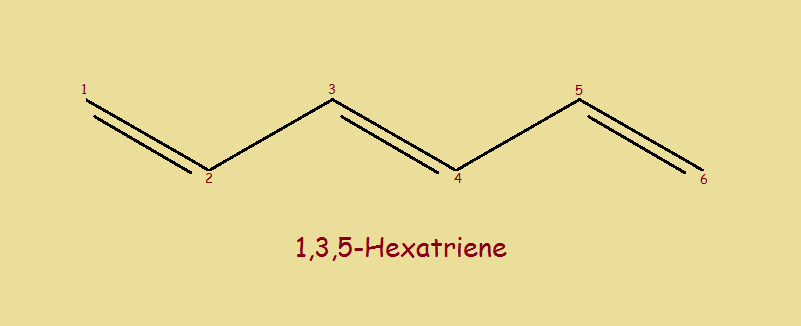
Carbon and hydrogen usually bonds in one of three ways: single bonds (-), double bonds (=), and triple bonds (≡). In this article, we will feature only single and double carbon-to-carbon bonds… two single and three double. The compound is 1,3,5-hexatriene. Notice the featured image (above) that illustrates the most common way it is drawn.
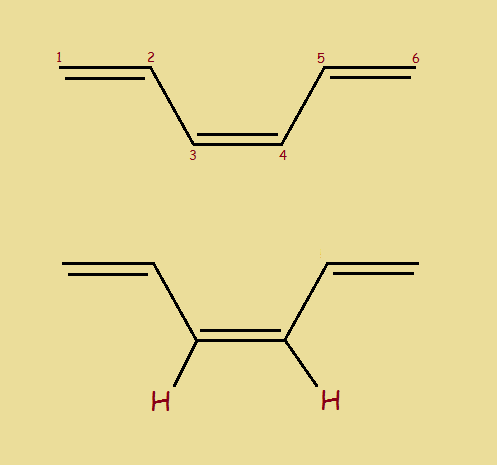
Another Isomer
There is another way to draw 1,3,5-hexatriene. It represents a second form, an isomer1, of the first form. We present a drawing of it, as well, to the right. Notice, however, it is drawn twice. Once illustrating two hydrogen atoms relevant to our discussion. The two isomers differ somewhat in chemical behavior.
Isomer I Revisited
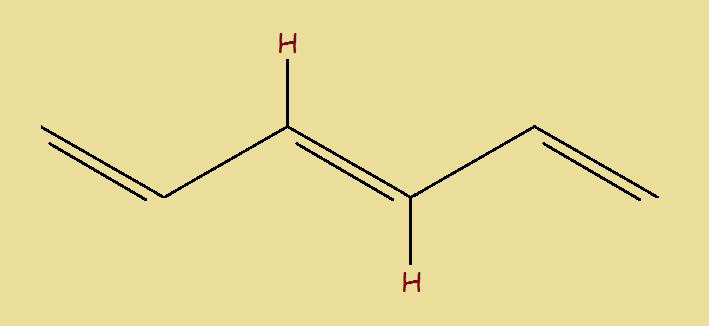
We now present, once again, our first isomer of 1,3,5-Hexatriene… this time with its relevant hydrogen atoms. We’ll call it Isomer I. Notice in this drawing, the two hydrogen atoms appear on opposite sides of the (3,4) double bond. Isomer II (for we now so call it) had both hydrogen atoms on the same side of the (3,4) double bond.
Because of the difference and for the purpose of identification, we will (at this point) call Isomer I trans-2-1,3,5-hexatriene. The prefix “trans” means across. The hydrogen atoms are across the double bond from each other.
We (again, for now) call Isomer II cis-1,3,5-hexatriene. The prefix “cis” means same side.
Out with the Old
The use of cis and trans, even though I just introduced it, has largely lost favor, being replaced by other notation (for reasons we won’t discuss). It should be noted that the old-school terminology is dying slowly. The new notation is the accepted IUPAC2 official notation.
In with the New
Rather than read about the new notation, we figured a video would be an invigorating change. We here embed a Khan Academy video (4:55) for your enjoyment.
Alkene Isomers – The Fun Begins
So the names of these two isomers are:
(E)-1,3,5-hexatriene
and
(Z)-1,3,5-hexatriene
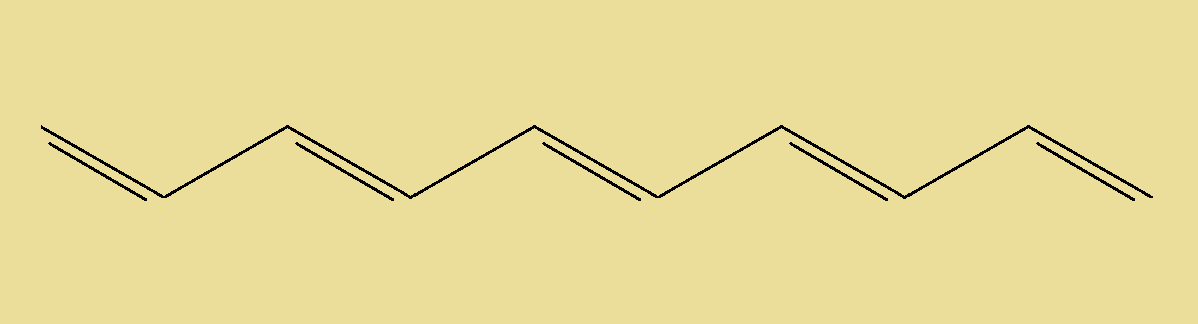
As you might imagine, the fun begins when there is a longer carbon chain containing more double bonds. As a simple example, consider the molecule seen at right. Considering the 2nd, 3rd and 4th double bonds’ configuration possibilities, how many alkene isomers do you believe exist? And if you figured that out, how would you specifically name each one?
1 Isomer: a compound with the same formula, but a different arrangement of the atoms
2 International Union of Pure and Applied Chemistry
Note: You might also enjoy The Difference Between Alkanes, Alkenes, and Alkynes
References:

I used to enjoy organic chemistry in university and the isomers were interesting. My chemistry partner in practicals was male and he was good at the maths while I was good at the shapes, so this benefited us both.