 Aluminum is a versatile metal, yet in order to be used in certain applications, it needs to be modified, improved. One way of modifying it is to add certain ingredients, such as a trace of copper, to toughen it. Another modification is the process of anodizing aluminum. What does that refer to?
Aluminum is a versatile metal, yet in order to be used in certain applications, it needs to be modified, improved. One way of modifying it is to add certain ingredients, such as a trace of copper, to toughen it. Another modification is the process of anodizing aluminum. What does that refer to?
Anode & Cathode
We’re all familiar with electroplating. We may have eaten meals using silver-plate utensils. Or we may have attended a classic auto show in which older cars have chrome-plated bumpers.
A metal coating (plating) from a chemical bath is applied using electricity to transfer the metal from a supply source (the anode) to the object to be plated (the cathode). The simplified artwork below illustrates a copper bath, complete with anode, cathode, and plating solution.
A marvelous thing happens: neutral copper atoms are given negative charges at the anode. These charged atoms enter the plating solution and are drawn to the positive cathode, to be deposited on its surface. This is the desired metal plate. But a second thing that can happen. What is that?
The Second Occurrence
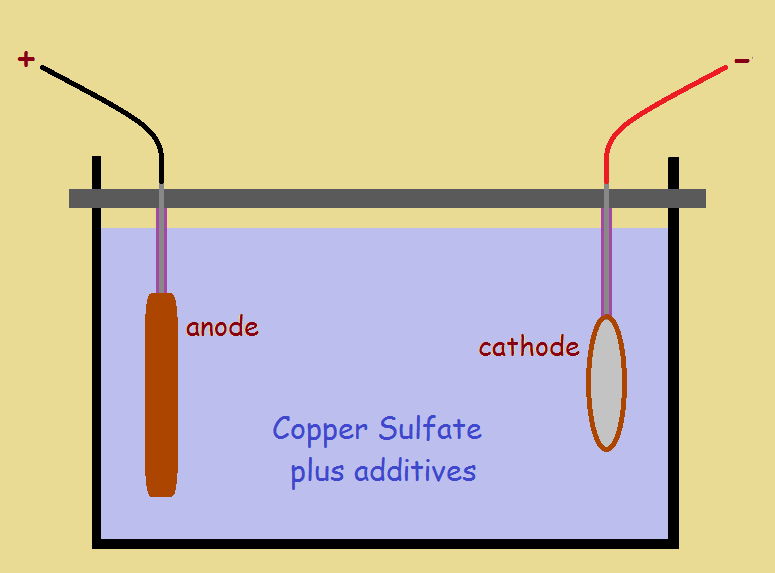
Another usually undesirable event can occur. If an electric current higher than bath specifications is supplied, some water molecules in a bath can break apart into hydrogen and oxygen ions. Positive hydrogen ions H+ are attracted to the cathode, where they combine to produce hydrogen gas H2 and escape to the surface.
Likewise, negative oxygen ions O-2 are attracted to the anode, where they may combine to form oxygen atoms, which also escape to the surface (but not before they unite to form molecules), these oxygen ions may accomplish something else, given the correct circumstances.
Those correct circumstances enable anodization!
A Bath Suitable for Anodizing Aluminum
An alloy of aluminum such as 6061 aluminum is anodized using a 20% sulfuric acid bath that is strictly maintained at 0oC. Generally, it is best to operate the power source in a constant current mode, not a constant voltage mode. The current needed to accomplish the task depends upon the surface area of the piece.
If the area is in square feet, the current density needed is approximately 15 ASF (amps per square foot). During anodization, the voltage will rise as the process approaches completion. A final of 75 volts should suffice for most applications. The color of the piece will pass through shades of charcoal gray.
But What Is It All About?
The aluminum anode is immersed in the cold sulfuric acid bath. The cathode is made of a non-dissolving substance: perhaps niobium coated titanium. When the current is applied, the aluminum anode does not dissolve. All of the electric current goes toward producing hydrogen and oxygen ions.
The aluminum, with no metal to plate on its surface, receives oxygen ions only. These ions are extremely reactive. They rapidly combine with the surface of the aluminum, producing a thick coating of aluminum oxide, Al2O3.
Aluminum, untreated, naturally forms an oxide layer by itself. However, the anodizing process greatly increases its thickness. So let’s ask and answer a list of questions that will explain what is going on…
Aluminum Vs Anodized Aluminum

Aluminum metal and aluminum with its normal thin-skin of oxide is electrically conductive. And like other metals, it also conducts heat. Interestingly, anodized aluminum (we speak hear of so-called hard-anodized or black-anodized aluminum, described above) still conducts heat, but it resists electricity!
There is another benefit to anodizing aluminum… Instead of being relatively soft like pure aluminum, anodized aluminum is scratch-resistant. Why is that? Well, the coating is, after all, a thick film of aluminum oxide. Why does that impart hardness?
Well, consider this: some of our most precious stones, including sapphire, are either high in or nearly pure aluminum oxide. Sapphire has a MOHS scale hardness of 9.0. Ditto for ruby. Is that impressive? It should be. Aluminum metal’s MOHS hardness is just 2.75!
Scratch resistance is very valuable in decorative objects. But the author of this article recalls one application he was involved with that involved radio astronomy.
Extra Credit: Anodized Aluminum in Radio Astronomy

Radio astronomy involves the processing of radio-frequency signals received from objects in space. Those signals are incredibly weak, as one might expect of a signal traveling light years! In order to be properly interpreted, those signals must be carefully received and amplified.
Cryogenically cooled, “low noise” pre-amplifiers and amplifiers must be used to accomplish the task. Electrical isolation is important, to avoid stray signals, while heat conductivity is maintained. Why is heat conductivity important?
Since pre-amps and amps need to be cooled cryogenically, they must be isolated from the outside world. Anodized connections accomplish the task. They allow the heat produced to be taken away, as they act as bridges between the devices and cryogenic coolant.
Note: You might also enjoy Use of the Hull Cell in Maintaining Electroplating and Electroforming Baths
References:

Just a note: Mohs is not an abbreviation or acronym – it’s the name of the man (Friedrich Mohs) who originated that hardness scale. So just the M should be capitalized, not the other letters. (Writing “on Mohs’s scale” is perfectly correct but sounds like Moses so it’s not much good; “on Mohs’ scale” is bad grammar so that can’t work; therefore the usual best solution is to write “on the Mohs scale” – it has good grammar, correct spelling, and no accidental resemblances.)
I read,”As the reciprocal of one ohm, it is the word ohm spelled backwards, at the suggestion of Sir William Thomson (Lord Kelvin) in 1883.” Oh, Mhos’ would be proper English. Just as Jesus’ and Moses’ is correct. Jesus’s and Moses’s is not. What I’ve written here is not to be smart-alecky or rude.